Information
Journal Policies
ARC Journal of Immunology and Vaccines
Volume-1 Issue-1, 2016
Abstract
HBV is a member of Hepadnavirus genus. HBV is a DNA virus with a diameter of 42 nm consisting of an outer lipid envelope which contains proteins HBsAg, pre-S1 and pre-S2. HBsAg is a product of ‘s’ gen. The s geneinvolves ‘a’ epitope- 124. -147. codones and this region is the affecting region for anti-HBs antibody binding. Vaccine /Immunglobulin escape mutations occur at HBV DNA s gene’s ‘a determinant’ region, 127-149. codones. These mutations causes inability to bind of anti HBs antibody to HBsAg. Also, these variants are transmissible among humans although individuals were vaccined. The result of our literature investigation, the number of the cases which caused by vaccine escape mutant variants were found to be increased, especially in high endemicity region. Because of that, we think vaccine escape mutants are growing very important public health problem.
2.KEYWORDS
3. INTRODUCTION
4.HBV VIROLOGY
5.LITERATURE REVIEW
6.CONCLUSION
7.REFERENCES
AUTHOR DETAILS
Muge Ozguler1, Murat Sayan2,3
1Elazıg Education and Research Hospital, Infectious Diseases and Clinical Microbiology Department, Elazıg, Turkey.
2Kocaeli University, Medical Faculty Clinical Laboratory, PCR Unit Izmit, Kocaeli, Turkey.
3Research Center of Experimental Health Sciences, Near East University, Nicosia, Northern Cyprus.
[email protected], [email protected].
KEYWORDS
Hepatitis B virus infection; Vaccine escape;Hepatitis Bsurface antigen mutants.
INTRODUCTION
Hepatitis B virus is a DNA virus which is a member of hepadnaviridae. It is responsible nearly 300 million chronic HBV infection and over 1 million deaths per year due to HBV-related end-stage liver disease, liver cirrhosis and liver cancer.HBV viruses can be present all of the body fluids and blood of the infected person. So, it can be transmitted such as perinatally, sexually, blood transfusions, unsafe injection, injecting drug with using mutual syringes and occupational exposure of health care workers [1].
Currently, HBV viruses have been classified into 10 genotypes [A-J]which can be further sub-divided into over 40 sub-genotypes [2-4].Genotype D and subgenotype D1 HBV virus infections are common in Turkey[5,6,7].
The worldwide prevalence of chronic HBV infection in the general population is 5%, but it differs from one geographical area to another, example 0.1%-2.0% in the United States and Western Europe, 2.0%-8.0% in Eastern Mediterranean countries [such as Turkey] and Japan, and 5.0%-20.0% in South-Eastern Asia and sub-Saharan Africa.In highly endemic areas the majority of chronic carriers acquire HBV infection at birth or in the first decade of life, whereas in countries with a low endemicity, HBV transmission occurs mostly in adulthood due to unsafe sexual contact, using mutual syringes or parenteral exposure to contaminated medical equipment or blood transfusion[8].
Because of it’s caused diseases, some strategies have been devoloped to fight HBV infection such as treating the chronically infected patients as much as possible, preventing the transmission and immunizing susceptible individuals.Among them, vaccination is the most effective by preventing individuals from contracting HBV infection[9]. Thirty years ago the first HBV vaccine was presented to use in fight against HBV. The vaccine contains highly antigenic the subviral particle”; HBsAg [10].
HBV vaccines have highly antigenic effect on immun system. It cause creating antibodies against to HBV surface antigen [HBsAg]directly.HBsAg is a product of ‘s’ gen. The ‘a’ epitope involves 124. -147. codones and this region is the affecting region for anti-HBs antibody binding. The mutations in this area can cause conformational changes at HBsAg and create ‘vaccine escape mutant variants’[11]. This situation is a public health threat and these mutants can cause infections at vaccined individuals. Such mutant variants may be dominant variant HBV virus in future. The aim of this review is providing attention to this point.
HBV VIROLOGY
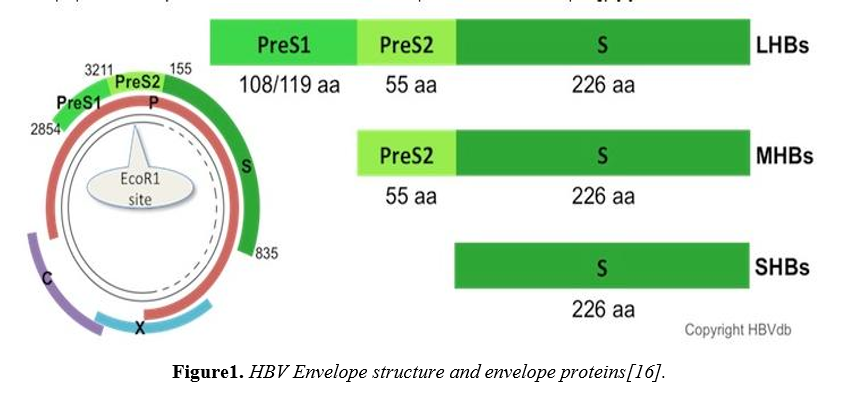
HBV is a member of Hepadnavirus genus. HBV is a DNA virus with a diameter of 42 nmol/L consisting of an outer lipid envelope which contains proteins HBsAg, pre-S1 and pre-S2 . These are provide the viral binding, entry into the hepatocytes [12,13].
The HBV genome consists of ~3.2 kb, 3200 base pairs ofpartially double-stranded circular DNA containing four[P, C, S and X] overlapping open reading frames [ORF]with a nucleotide diversity of ≥ 8% in different genotypes[14].The core [C] gene encodes the capsid protein, HBcAg, which is the major structural protein of the nucleocapsid. The preC/C ORF is transcribed into a precore/core fusion protein. The X gene gives rise to a protein of not completely understood function but it has transactivating functions. The P gene codes for the viral polymerase/reverse transcriptase.In addition, the HBV polymerase [pol] gene completely overlaps with the envelope [S] gene.The three envelope proteins are encoded by a single ORF with three in frame start codons. Consequently, they share a common C-terminus that provides the membrane anchor and differ in length at their N-termini. They are designated the Large [L], Middle [M-] and Small [S-] surface protein, respectively, with the first-mentioned playing the major role in this proposal. Compared to the S-protein [the classic HBs-Ag], the M-protein is extended to the 55 preS2- and the L-protein additionally to the 108 preS1-encoded amino acids [15].
Currently the primary element for diagnosis and target of immunoprophylaxis of HBV infection is HBsAg. The dominant epitopes of HBsAg, which are the targets of neutralizing B-cell responses, reside in the ‘‘a’’ determinant [aa 124-147] within the major hydrophilic region [MHR]. It is in a form of two major and one minor loops with cysteine-disulphide bonds, protruding from the outer surface of the virus. The second hydrophilic loop [aa 139 to 147 or 149] is the major target for neutralizing anti-HBs produced following natural infection or vaccination[17].The most common pattern mutations are sP120T, sS143L, sD144A/E, sE164D, sL127P, sG129H, sM133I/T, sP/T134A/L, sS140L, sG145A/R, sW172, and sW182 in the literature [18]. But the variant sG145R was identified mostly as a vaccine escape mutant in these literature [54%]. It has been demonstrated that it is also associated with HBV-related chronic liver disease in non-vaccinated subjects[19]. On the other hand, the horizontal transmission of the sG145R mutant strain has been reported[20].Naturally occurring surface gene variants have also been reported around the world in persons who have not been immunized[21,22].
The ‘a’ determinant is common all genotypes. Neutrolizing antiHBs response which vaccine caused,provides protection against all HBV genotypes andsubtypes.Alterations in this region of the surface antigen can determine conformational changes thatcan allow replication of mutated viruses in vaccinated people [vaccine escape mutants or VEMs][23].
LITERATURE REVIEW
Currently, we can use five oral anviral regimens [Lamivudine, Telbuvidine, Adefovir, Entecavir, Tenofovir] and pegile interferons subcutaneously for the treatment of chronic hepatitis B. Especially, low potential antiviral drugs are responsible of drug resistance at p genes. Because of s genes and p genes overlap, a mutation at p genes, especially mutual region for p and s genes, can effect eachother.
So, drug associated p gene mutations, can cause s gene mutant variants. If mutation occurs at ‘a determinant’ of s genes, the mutation effects the response to vaccine. Out of this regions,occurred mutations in the area which including 161, 164, 172, 173, 175, 176, 182, 193, 194, 195, 196. codones also cause vaccine escape mutations [24].In addition, the mutations in "a" epitope correlate with the absence of detectable anti-HBsAg. These mutations also called as Antiviral Drug Associated Potential Vaccine Escape Mutation-ADAPVEM at the patient who received orally antiviral drugs previously.
The mutation occured during antiviral treatment cause NUC resistances. These mutations can cause changes in the overlapping reading frame and alter the C-terminal region of HBsAg [25]. LAM-resistant HBV [rtV173L + rtL180M + rtM204V] has been shown to cause two amino acid changes in the overlapping surface gene [sE164D + sI195M],and cause reduction at anti-HBs binding similar with the vaccine escape mutant sG145R [34, 35]. This situation significantly reduces antiHBs binding due to changes in the HBsAg and effects vaccine and HB-Ig response [18]. As a result, antiviral drug resistance can effect the HBsAg protein structure and anti HBs binding in these patients. ADAPVEM’s are important potential threat for public health. Because these mutant variants can be dominant variant among the HBV viruses and it can be transmissible an individual to another although the vaccinewas applied[26].
Since 1990, vaccine escape mutant has been reported as an important public health threat. A literature investigation about vaccine escape mutation [VEMs] was done from 1990 to 2015. We obtained total 69reports about vaccine escape mutant HBV variants. Turkey has nine reports[18,24,26-32]. China has eight reports[33-40]and Taiwan has seven reports[41-47]. England have five reports[48-52]. Thailand, Japan, Italy USA, India, Iran have four reports from each country[53-76]. France has three reports[77-79]. Argentina have two reports[79-81] and the other countries have one report from each country total 11 reports[82-92].
Firstly, an Italian infant has been established as having a vaccine escape mutant [G145R] variants [61]. In 1990-2000 years total 14 report, in 2001-2010 years total 30 report and in 2011-2015 years 24 report.Most of the reports were including G145A/R mutations and most of the reports were from high endemicity regions.
It was observed that, this problem is growing too fast in the world wide. Especially in the countries which are situated in high endemicity region such as China, Thailand, Taiwan, China.Monitoring for more than a decade in these countries has shown that hepatitis B immunization programmes have increased the incidence of HBV vaccine escape variants [35,54].
In China, the prevalence of "α" determinant mutants in the children increased from 6.5% in 1992 to 14.8% in 2005, where the G145R mutant occurred most frequently. In contrast, mutation frequencies showed little difference between 1992 [9.4%] and 2005 [9.9%] in adults[93].
Similarly, in Taiwan, the prevalence of hepatitis B surface gene a determinant mutants increased from 7.8% before the vaccination program, to 19.6%, 28.1% and 23.1% at 5, 10 and 15 years after the program[44].
Turkey is an middle endemicity area [2–7%]. A recent study in Turkey found an overall prevalence of 4.19% for HBsAg, but the prevalence rates are highest in Southeastern Turkey [9–11%]. In 1997, all of the new borns were vaccined according to universal vaccination program of WHO and CDC in the Worldwide. By 1998, vaccination against to HBV was started in Turkey[94]. In Turkeyinterest in this subject is increasing every day. Especially, Sayan contributesmany studies to Turkish literature in this subject.
First HBV vaccine escape mutation [sT143stop codon] was observed in Turkey in a child with CHB[27].After that,Ozaslan et al.[28]sequencing the amplified surface gene region has suggested sM125T and sT127P mutations as HBsAg escape mutations in Turkish patients with CHB and their family members.They had investigated 40 HBV DNA positive patients among 132 HBsAg carriers.Ten kinds of point mutations had been identified within the S region. The highest rates of mutation had been obtained in chronic hepatitis patients and their family members. The amino acid mutations 125 [M -> T] and 127 [T -> P] had been found on the first loop of 'a'-determinant [28]. Another study has been described a diagnostic HBsAg escape mutation [sS143L, sQ101H, sS117N, sT118R, sP120Q ] causing chronic HBV infection in a previously vaccinated treatment-naı¨ve Turkish patient[29].
The most comprehensive study about this subject is Sayan ‘s study in Turkey. Sayan et al [18]had evaluated 142 patients who are undergoing treatment and 185 patient who are treatment naive. The sP120T, sG130R, sM133I, sY134N, sD144E, sS143L and sG145R mutations had been obtainedin their study. The prevalence of typical HBsAg escape mutations had beendetected treatment naive and treatment groups 8.1% and 8.5% respectively. In both groups sG145R escape mutation had been observed. The sG145R escape mutation prevalence had been detected at a low frequency [1.2%] in Turkish patients with CHB.
Sayan et al. [24] also had been evaluated 94 patients who HBsAg-positive and having hemodialysis. They had been observed ADAPVEM mutations which located in 161, 164, 172, 173, 175, 176, 182, 193, 194, 195, 196. codones at 43 patients of these 94 hemodialysis patients.
A case report who has sS143T, sD144E, sG145R, sE164D and sI195M mutations all together.had been presented by Sayan recently. It was emphasized that the patient as a first case including G145R mutation in Turkey[37].
Kaymakoğlu et al. [31] reported a case about an acute hepatitis B caused by immune escape variants in the absence of any immunosuppression or cytotoxic chemotherapy.
Also, we evaluated recently a case who experienced with pegile interferon, lamivudine and entecavir 0.5 mg and 1 mg. Despite treatment his viral load increased, we applied sequence analysing and observed T127P vaccine escape mutations[32]
CONCLUSION
Vaccine /Immunglobulin escape mutations occur at HBV DNA s gene’s ‘a determinant’ region, 127-149. and 161-195codones. It can be a result of previous NUC treatment. These mutant variants are capable to prevent anti HBs binding. Also, these variants are transmissible among humans. Because of that, ADAPVEM formation should be monitored. In addition, such mutated viruses can be undetectable by the current diagnosticassays. Because ot that, we think, VEMs are growing very important public heath problem. Required precaution methods should be taken immediately. New vaccines development studies should be done as soon as possible.
REFERENCES
- Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol 2005; 34 (1):S1-S3
- Locarnini S, Littlejohn M, Aziz MN, Yuen L. Possible origins and evolution of the hepatitis B virus (HBV). Semin Cancer Biol 2013;23:561–575.
- Schaefer S. Hepatitis B virus taxonomy and hepatitis B virus genotypes. World J Gastroenterol 2007;13:14–21.
- Coppola N, Onorato L, Minichini C, Di Caprio G, Starace M, Sagnelli C, Sagnelli E. Clinical significance of hepatitis B surface antigen mutants.World J Hepatol. 2015;7(27):2729-2739.
- Sunbul M, Leblebicioglu H. Distribution of hepatitis B virus genotypes in patients with chronic hepatitis B in Turkey. World J Gastroenterol. 2005;11:1976-1980.
- Sunbul M. Hepatitis B virus genotypes: Global distribution and clinical importance. World J Gastroenterol. 2014; 20(18): 5427-5434.
- Sayan M, Dogan C. Genotype/subgenotype distribution of hepatitis B virus among hemodialysis patients with chronical hepatitis B. Ann Hepatol. 2012;11(6):849-54.
- Sagnelli E, Sagnelli C, Pisaturo M, Macera M, Coppola N. Epidemiology of acute and chronic hepatitis B and delta over the last 5 decades in Italy. World J Gastroenterol 2014; 20: 7635-7643
- Chen DS. Hepatitis B vaccination: The key towards elimination and eradication of hepatitis B. J Hepatol 2009;50:805–816.
- Szmuness W, Stevens CE, Harley EJ, Zang EA, Oleszko WR, William DC, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med 1980;303:833–841.
- Valenzuela P, Medina A, Rutter WJ, Ammerer G, Hall BD. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature 1982;298:347–350.
- Coppola N, Sagnelli C, Pisaturo M, Minichini C, Messina V, Alessio L, Starace M, Signoriello G, Gentile I, Filippini P, Sagnelli E. Clinical and virological characteristics associated with severe acute hepatitis B. Clin Microbiol Infect 2014; 20: O991-7.
- Harrison T. Desk Encyclopedia of General Virology. Boston: Academic Press, 2009: 455.
- Sagnelli C, Ciccozzi M, Pisaturo M, Lo Presti A, Cella E, Coppola N, Sagnelli E. The impact of viral molecular diversity on the clinical presentation and outcome of acute hepatitis B in Italy. New Microbiol 2015; 38: 137-147.
- Torresi J. The virological and clinical significance of mutations in the overlapping envelope and polymerase genes of hepatitis B virus. J Clin Virol 2002;25:97–106.
- Bruss V .Envelopment of the hepatitis B virus nucleocapsid. Virus Res, 2004;106(2):199-209.
- Coppola N, Onorato L, Minichini C, Di Caprio G, Starace M, Sagnelli C, et al. Clinical significance of hepatitis B surface antigen mutants. World J Hepatol 2015; 7(27): 2729-2739.
- Sayan M, Sentürk O, Akhan SÇ, Hülagü S, Cekmen MB. Monitoring of hepatitis B virus surface antigen escape mutations and concomitantly nucleos(t)ide analog resistance mutations in Turkish patients with chronic hepatitis B. Int J Infect Dis. 2010;14(3):e136-41.
- OonC.J, W.N. Chen. Current aspects of hepatitis B surface antigen mutants in Singapore. J Viral Hepat 1998;5:17–23.
- ThakurV., KazimS.N., GuptanR.C.,. Hasnain, S.E. BartholomeuszA, MalhotraV., Sarin S.K. Transmission of G145R mutant of HBV to an unrelated contact. J Med Virol 2005;76: 40–46.
- CooremanM.P, van RoosmalenM.H., RMorsche.T., SunnenC.M., de VenE.M.,. JansenJ.B, et al. Characterization of reactivity pattern of murine monoclonal antibodies against wild-type hepatitis B surface antigen to G145R and other naturally occurring ‘a’ loop escape mutations. Hepatology 1999:30; 1287–1292.
- HouJ., WangZ., ChengJ., LinJ., LauG.K., SunJ., et al. Prevalence of naturally occurring surface gene variants of hepatitis B virus in non-immunised surface antigen-negative Chinese carriers Hepatology 2001;34:1027–1034.
- Romanò L, Paladini S, Galli C, Raimondo G, Pollicino T, Zanetti AR. Hepatitis B vaccination.Hum Vaccin Immunother. 2015;11(1):53-7.
- Sayan M, Cavdar C, Dogan C. Naturally occurring polymerase and surface gene variants of hepatitis B virus in Turkish hemodialysis patients with chronic hepatitis B. Jpn J Infect Dis. 2012;65(6):495-501.
- Bartholomeusz A., Locarnini S., Hepatitis B virus mutations associated with antiviral therapy.J Med Virol 2006;78:52–55.
- Sayan M, Akhan SC. Antiviral drug-associated potential vaccine-escape hepatitis B virus mutants in Turkish patients with chronic hepatitis B. Int J Infect Dis. 2011;15(10):722-6.
- Kutlu T., Soycan L.Y., Karataylı E., Turkyılmaz A.R., Yurdaydın C., Bozdayı M. The first identified hepatitis B virus vaccine escape mutation in Turkey. Letter to the Editor. J Clin Virol, 2006;35: 201–202.
- Ozaslan M., Ozaslan E., Barsgan A., Koruk M..Mutations in the S gene region of hepatitis B virus genotype D in Turkish patients. J Genetics 2007; 86:195–201.
- Sayiner A.A., Agca H., Sengonul A., Celik A., Akarsu M.A new hepatitis B virus vaccine escape mutation in a renal transplant recipient. J Clin Virol 2007;38:157–160.
- Sayan M, Buğdacı MS. HBV vaccine escape mutations in a chronic hepatitis B patient treated with nucleos(t)ide analogues. Mikrobiyol Bul 2013; 47: 544-549.
- Kaymakoglu S, Baran B, Onel D, et al. Acute hepatitis B due to immune-escape mutations in a naturally immune patient. Acta Gastroenterol Belg 2014; 77: 262-265.
- Özgüler M., Sayan M. [Vaccine Escape Mutation due to Using Nucleos(t)id Analog at Chronic Hepatitis B: A Case Report]. F.Ü.Sağ.Bil.Tıp Derg. 2015; 29 (2):87-89.[Turkish]
- Ho MS, Lu CF, Kuo J, Mau YC, Chao WH. A family cluster of an immune escape variant of hepatitis B virus infecting a mother and her two fully immunized children. Clin Diagn Lab Immunol. 1995;2(6):760-2.
- Ni F1, Fang D, Gan R, Li Z, Duan S, Xu Z. A new immune escape mutant of hepatitis B virus with an Asp to Ala substitution in aa144 of the envelope major protein. Res Virol. 1995;146(6):397-407.
- He C, Nomura F, Itoga S, Isobe K, Nakai T. Prevalence of vaccine-induced escape mutants of hepatitis B virus in the adult population in China: a prospective study in 176 restaurant employees. J Gastroenterol Hepatol. 2001;16(12):1373-7.
- Huang X, Lu D, Ji G, Sun Y, Ma L, Chen Z, Zhang L, Huang J, Yu L. Hepatitis B virus (HBV) vaccine-induced escape mutants of HBV S gene among children from Qidong area, China. Virus Res. 2004;99(1):63-8.
- Hu Q, Huang JG, Lei YC, Huang HP, Yang Y, Yang DL. [Detection of mutants of the "a" determinant region of hepatitis B surface antigen S gene among Wuhan childhood patients]. Zhonghua Gan Zang Bing Za Zhi. 2005;13(8):594-6. Chinese.
- Shi Y, Wei F, Hu D, Li Q, Smith D, Li N, Chen D.Mutations in the major hydrophilic region (MHR) of hepatitis B virus genotype C in North China. J Med Virol. 2012;84(12):1901-6.
- Su H, Zhang Y, Xu D, Wang B, Zhang L, Li D, Xiao D, Li F, Zhang J, Yan Y. Occult hepatitis B virus infection in anti-HBs-positive infants born to HBsAg-positive mothers in China. PLoS One. 2013: 8(8):e70768.
- Wang XY, Harrison TJ, He X, Chen QY, Li GJ, Liu MH, Li H, Yang JY, Fang ZL. The prevalence of mutations in the major hydrophilic region of the surface antigen of hepatitis B virus varies with subgenotype. Epidemiol Infect. 2015;143(16):3572-82.
- Chiou HL, Lee TS, Kuo J, Mau YC, Ho MS. Altered antigenicity of 'a' determinant variants of hepatitis B virus. J Gen Virol. 1997;78 ( Pt 10):2639-45.
- Lee PI, Chang LY, Lee CY, Huang LM, Chang MH. Detection of hepatitis B surface gene mutation in carrier children with or without immunoprophylaxis at birth. J Infect Dis. 1997;176(2):427-30.
- Hsu HY, Chang MH, Liaw SH, Ni YH, Chen HL. Changes of hepatitis B surface antigen variants in carrier children before and after universal vaccination in Taiwan. Hepatology. 1999;30(5):1312-7.
- Hsu HY, Chang MH, Ni YH, Chen HL. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut. 2004;53(10):1499-503.
- Hsu HY, Chang MH, Ni YH, Chiang CL, Chen HL, Wu JF, Chen PJ.No increase in prevalence of hepatitis B surface antigen mutant in a population of children and adolescents who were fully covered by universal infant immunization. J Infect Dis. 2010 Apr 15;201(8):1192-200.
- Lai MW, Lin TY, Tsao KC, Huang CG, Hsiao MJ, Liang KH, Yeh CT. Increased seroprevalence of HBV DNA with mutations in the s gene among individuals greater than 18 years old after complete vaccination. Gastroenterology. 2012;143(2):400-7.
- Lin YM, Jow GM, Mu SC, Chen BF. Naturally occurring hepatitis B virus B-cell and T-cell epitope mutants in hepatitis B vaccinated children. Scientific World Journal. 2013;2013:571875
- Harrison TJ, Hopes EA, Oon CJ, Zanetti AR, Zuckerman AJ. Independent emergence of a vaccine-induced escape mutant of hepatitis B virus. J Hepatol. 1991;13(4):105-7.
- Ngui SL1, O'Connell S, Eglin RP, Heptonstall J, Teo CG. Low detection rate and maternal provenance of hepatitis B virus S gene mutants in cases of failed postnatal immunoprophylaxis in England and Wales. J Infect Dis. 1997;176(5):1360-5.
- Ijaz S, Torre F, Tedder RS, Williams R, Naoumov NV. Novel immunoassay for the detection of hepatitis B surface 'escape' mutants and its application in liver transplant recipients. J Med Virol. 2001;63(3):210-6.
- Seddigh-Tonekaboni S1, Lim WL, Young B, Hou JL, Waters J, Luo KX, Thomas HC, Karayiannis P. Hepatitis B surface antigen variants in vaccinees, blood donors and an interferon-treated patient. J Viral Hepat. 2001;8(2):154-8.
- Larralde O, Dow B, Jarvis L, Davidson F, Petrik J. Hepatitis B escape mutants in Scottish blood donors. Med Microbiol Immunol. 2013;202(3):207-14.
- Poovorawan Y, Theamboonlers A, Chongsrisawat V, Sanpavat S. Molecular analysis of the a determinant of HBsAg in children of HBeAg-positive mothers upon failure of postexposure prophylaxis. Int J Infect Dis. 1998;2(4):216-20.
- Theamboonlers A, Chongsrisawat V, Jantaradsamee P, Poovorawan Y. Variants within the "a" determinant of HBs gene in children and adolescents with and without hepatitis B vaccination as part of Thailand's Expanded Program on Immunization (EPI). Tohoku J Exp Med. 2001;193(3):197-205.
- Sa-Nguanmoo P, Tangkijvanich P, Tharmaphornpilas P, Rasdjarmrearnsook AO, Plianpanich S, Thawornsuk N, Theamboonlers A, Poovorawan Y. Molecular analysis of hepatitis B virus associated with vaccine failure in infants and mothers: a case-control study in Thailand. J Med Virol. 2012;84(8):1177-85.
- Yimnoi P, Posuwan N, Wanlapakorn N, Tangkijvanich P, Theamboonlers A, Vongpunsawad S1, Poovorawan Y1. A molecular epidemiological study of the hepatitis B virus in Thailand after 22 years of universal immunization. J Med Virol. 2015;31. doi: 10.1002/jmv.24368.
- Okamoto H, Yano K, Nozaki Y, Matsui A, Miyazaki H, Yamamoto K, Tsuda F, Machida A, Mishiro S. Mutations within the S gene of hepatitis B virus transmitted from mothers to babies immunized with hepatitis B immune globulin and vaccine. Pediatr Res. 1992;32(3):264-8.
- Yamamoto K, Horikita M, Tsuda F, Itoh K, Akahane Y, Yotsumoto S, Okamoto H, Miyakawa Y, Mayumi M. Naturally occurring escape mutants of hepatitis B virus with various mutations in the S gene in carriers seropositive for antibody to hepatitis B surface antigen. Virol. 1994;68(4):2671-6.
- Shinji T, Koide N, Hanafusa T, Hada H, Oka T, Takayama N, Shiraha H, Nakamura M, Ujike K, Yumoto Y, Tsuji T. Point mutations in the S and pre-S2 genes observed in two hepatitis B virus carriers positive for antibody to hepatitis B surface antigen. Hepatogastroenterology. 1998;45(20):500-2.
- Ishigami M,, Honda T, Ishizu Y, Onishi Y, Kamei H, Hayashi K, Ogura Y, Hirooka Y, Goto H. Frequent incidence of escape mutants after successful hepatitis B vaccine response and stopping of nucleos(t)ide analogues in liver transplant recipients. Liver Transpl. 2014;20(10):1211-20.
- Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman AJ, Thomas HC. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336(8711):325-9.
- Mele A, Tancredi F, Romanò L, Giuseppone A, Colucci M, Sangiuolo A, Lecce R, Adamo B, Tosti ME, Taliani G, Zanetti AR. Effectiveness of hepatitis B vaccination in babies born to hepatitis B surface antigen-positive mothers in Italy. J Infect Dis. 2001;184(7):905-8.
- Mascagni P, Romanò L, Scanziani R, Toffoletto F. [Characterisation of an HBsAg mutant of hepatitis B virus (HBV) isolated from a dialysed patient involved in an occupational accident]. Med Lav. 2005;96(3):231-7.
- Sticchi L, Caligiuri P, Cacciani R, Alicino C, Bruzzone B.Epidemiology of HBV S-gene mutants in the Liguria Region, Italy: Implications for surveillance and detection of new escape variants. Hum Vaccin Immunother. 2013 Mar;9(3):568-71.
- Schirmer P, Winters M, Holodniy M.HIV-HBV vaccine escape mutant infection with loss of HBV surface antibody and persistent HBV viremia on tenofovir/emtricitabine without antiviral resistance. J Clin Virol. 2011;52(3):261-4.
- Laoi BN, Crowley B.Molecular characterization of hepatitis B virus (HBV) isolates, including identification of a novel recombinant, in patients with acute HBV infection attending an Irish hospital. J Med Virol. 2008;80(9):1554-64.
- Foy MC, Thio CL, Hwang HS, Saulynas M, Hamilton JP, Fine DM, Atta MG. False-negative hepatitis B virus (HBV) surface antigen in a vaccinated dialysis patient with a high level of HBV DNA in the United States. Clin Vaccine Immunol. 2012;19(5):820-2.
- Anderson M, Gaseitsiwe S, Moyo S, Wessels MJ, Mohammed T, Sebunya TK, Powell EA, Makhema J, Blackard JT, Marlink R, Essex M, Musonda RM. Molecular characterisation of hepatitis B virus in HIV-1 subtype C infected patients in Botswana. BMC Infect Dis. 2015;13;15:335.
- Chakravarty R, Neogi M, Roychowdhury S, Panda CK. Presence of hepatitis B surface antigen mutant G145R DNA in the peripheral blood leukocytes of the family members of an asymptomatic carrier and evidence of its horizontal transmission. Virus Res. 2002;90(1-2):133-41.
- Datta S, Banerjee A, Chandra PK, Chowdhury A, Chakravarty R. Genotype, phylogenetic analysis, and transmission pattern of occult hepatitis B virus (HBV) infection in families of asymptomatic HBsAg carriers. J Med Virol. 2006;78(1):53-9.
- Kazim SN, Sarin SK, Sharma BC, Khan LA, Hasnain SE. Characterization of naturally occurring and Lamivudine-induced surface gene mutants of hepatitis B virus in patients with chronic hepatitis B in India. Intervirology. 2006;49(3):152-60.
- Velu V, Saravanan S, Nandakumar S, Dhevahi E, Shankar EM, Murugavel KG, Kumarasamy T, Thyagarajan SP. Transmission of "a" determinant variants of hepatitis B virus in immunized babies born to HBsAg carrier mothers. Jpn J Infect Dis. 2008;61(1):73-6.
- Moradi A, Zhand S, Ghaemi A, Javid N, Tabarraei A. Mutations in the S gene region of hepatitis B virus genotype D in Golestan Province-Iran. Virus Genes. 2012;44(3):382-7.
- Ghaziasadi A1, Alavian SM, Norouzi M, Fazeli Z, Jazayeri SM. Mutational analysis of HBs Ag-positive mothers and their infected children despite immunoprophylaxis. Iran J Allergy Asthma Immunol. 2013 Aug 28;12(4):352-60.
- Aghakhani A, Mohraz M, Aghasadeghi MR, Banifazl M, Vahabpour R, Karami A, Foroughi M, Ramezani A. Occult hepatitis B virus infection and S gene escape mutants in HIV-infected patients after hepatitis B virus vaccination. Int J STD AIDS. 2015 Sep 18. pii: 0956462415602419.
- Sadeghi A, Yahyapour Y, Poortahmasebi V, Shahmoradi S, Roggendorf M, Karimzadeh H, Alavian SM, Jazayeri SM. Clearance of HBV DNA in immunized children born to HBsAg-positive mothers, years after being diagnosed with occult HBV infection. J Viral Hepat. 2015 Nov 24. doi: 10.1111/jvh.12490.
- Soussan P, Pol S, Garreau F, Bréchot C, Kremsdorf D. Vaccination of chronic hepatitis B virus carriers with preS2/S envelope protein is not associated with the emergence of envelope escape mutants. J Gen Virol. 2001;82(Pt 2):367-71.
- Roque-Afonso AM, Ferey MP, Belkhiri D, Dussaix E. [HBs antigen mutants: prevalence, clinical and diagnostic implications]. Pathol Biol (Paris). 2005;53(8-9):563-8.
- Lada O, Benhamou Y, Poynard T, Thibault V. Coexistence of hepatitis B surface antigen (HBs Ag) and anti-HBs antibodies in chronic hepatitis B virus carriers: influence of "a" determinant variants. J Virol. 2006;80(6):2968-75.
- Cuestas ML, Mathet VL, Ruiz V, Minassian ML, Rivero C, Sala A, Corach D, Alessio A, Pozzati M, Frider B, Oubiña JR. Unusual naturally occurring humoral and cellular mutated epitopes of hepatitis B virus in a chronically infected argentine patient with anti-HBs antibodies. J Clin Microbiol. 2006;44(6):2191-8.
- Mathet VL, Cuestas ML, Ruiz V, Minassian ML, Rivero C, Trinks J, Daleoso G, León LM, Sala A, Libellara B, Corach D, Oubiña JR. Detection of hepatitis B virus (HBV) genotype E carried--even in the presence of high titers of anti-HBs antibodies--by an Argentinean patient of African descent who had received vaccination against HBV. J Clin Microbiol. 2006;44(9):3435-9.
- Awerkiew S, Däumer M, Reiser M, Wend UC, Pfister H, Kaiser R, Willems WR, Gerlich WH. Reactivation of an occult hepatitis B virus escape mutant in an anti-HBs positive, anti-HBc negative lymphoma patient. J Clin Virol. 2007;38(1):83-6.
- Szomor KN, Dencs A, Garai E, Rusvai E, Berencsi G, Takács M. Mutation spectra of the surface-protein-coding region of the HBV genome in HBV-vaccinated and non-vaccinated individuals in Hungary. Arch Virol. 2008;153(10):1885-92.
- Devesa M, Rodríguez C, León G, Liprandi F, Pujol FH. Clade analysis and surface antigen polymorphism of hepatitis B virus American genotypes. J Med Virol. 2004 Mar;72(3):377-84.
- Basuni AA, Butterworth L, Cooksley G, Locarnini S, Carman WF Prevalence of HBsAg mutants and impact of hepatitis B infant immunisation in four Pacific Island countries. Vaccine. 2004;22(21-22):2791-9
- Avellón A, Echevarria JM. Frequency of hepatitis B virus 'a' determinant variants in unselected Spanish chronic carriers. J Med Virol. 2006;78(1):24-36 .
- Baclig MO, Alvarez MR, Gopez-Cervantes J, Natividad FF. Unique surface gene variants of hepatitis B virus isolated from patients in the Philippines.J Med Virol. 2014 Feb;86(2):209-16.
- Al-Qudari AY, Amer HM, Abdo AA, Hussain Z, Al-Hamoudi W, Alswat K, Almajhdi FN. Surface Gene Variants of Hepatitis B Virus in Saudi Patients. Saudi J Gastroenterol. 2015 Oct 16. doi: 10.4103/1319-3767.167186.
- Virine B, Osiowy C, Gao S, Wang T, Castillo E, Martin SR, Lee SS, Simmonds K, van Marle G, Coffin CS. Hepatitis B Virus (HBV) Variants in Untreated and Tenofovir Treated Chronic Hepatitis B (CHB) Patients during Pregnancy and Post-Partum Follow-Up. PLoS One. 2015 Oct 16;10(10):e0140070. doi: 10.1371.
- Luongo M, Critelli R, Grottola A, Gitto S, Bernabucci V, Bevini M, Vecchi C, Montagnani G, Villa E. Acute hepatitis B caused by a vaccine-escape HBV strain in vaccinated subject: sequence analysis and therapeutic strategy. J Clin Virol. 2015;62:89-91.
- Oon CJ, Lim GK, Ye Z, Goh KT, Tan KL, Yo SL, Hopes E, Harrison TJ, Zuckerman AJ Molecular epidemiology of hepatitis B virus vaccine variants in Singapore. Vaccine. 1995;13(8):699-702.
- Lee KM, Kim YS, Ko YY, Yoo BM, Lee KJ, Kim JH, Hahm KB, Cho SW. Emergence of vaccine-induced escape mutant of hepatitis B virus with multiple surface gene mutations in a Korean child. J Korean Med Sci. 2001;16(3):359-62.
- Bian T, Yan H, Shen L, Wang F, Zhang S, Cao Y, Zhang S, Zhang Y, Bi S. Change in hepatitis B virus large surface antigen variant prevalence 13 years after implementation of a universal vaccination program in China. J Virol 2013; 87:12196-206.
- Tosun S. HBV vaccination in our country. Sted 2002; 11 (4);140-142. [Turkish].