Information
Journal Policies
The Paris System for Reporting Atypia in Urinary Cytology. A Study in a Retrospective Series
Francesc Alameda1,2*,Imma Soler1,Amparo Quinonero1,Emilia Romero1,Monica Bautista1,Belen LLoveras1,2
2.Universitat Autonòma de Barcelona.
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction: The diagnosis of atypia in urinary cytology is based on criteria with low reproducibility and consequently shows great inter-observer variability. The Paris System for Reporting Urinary Cytology (the Paris System, TPS) standardizes the cytological criteria used for the diagnosis of atypia and increases the reproducibility of this diagnosis.
Aim: To apply TPS to a retrospective series of urinary atypia cases and evaluate the results in comparison with follow-up during the two years after diagnosis.
Material and Methods: We reviewed 123 cases from how many patients? classified as atypical in urinary cytology during 2012 and 2013. Voided urine samples from all the cases were collected, processed on a Thin Prep platform and submitted to automatic reading using the Imager. Atypia was re-evaluated following TPS criteria.
Results: 52 cases had a positive biopsy in the follow-up. Considering TPS criteria as a number of cytological changes, when the cases fulfilled 3-4 cytological changes, the sensitivity of urine cytology for cancer was 89.7%, with low specificity. Considering the TPS criteria as indicated in the reporting (hierarchical changes), the sensitivity decreased to 33.3%, increasing the specificity
Conclusions: On the basis of our results, we propose that TPS be used as a number of criteria, non in hierarchical manner.
Urine cytology; Atypia, Follow-up, the Paris System,Public Health and Community Medicine
1. Introduction
Urinary cytology shows high sensitivity (70-95%) for high-grade urothelial carcinoma, with a specificity of around 95%. However, the sensitivity for low-grade carcinomas is varies between 10% and 70% depending on the series. Furthermore, there is considerable inter-observer variability [1-3].
The presence of atypical cells can be a problem that can raises patient management issues. Not all the authors agree to report atypia in urinary cytology because this diagnosis increases the number of cystoscopies with blank results [2]. However, some data favor the report of atypia, namely early tumor stage is related to initial positive or atypical cytology [4], tumor relapse can be detected if atypia is present in the cytological smear [4], and the diagnosis of atypia is associated with a high probability of progression to high-grade carcinoma in a short period of time, less than 1 year [5].
The criteria used to define cytological atypia are inaccurate, and the diagnosis is based more on the observer’s judgement than on objective criteria [6]. Furthermore, while the main aim of urinary cytology is the diagnosis of high-grade tumors [7], low-grade ones can also be diagnosed [2], and more than 50% of low-grade tumors diagnosed in biopsies show atypia in urine cytology [2].
Rosenthal et al. classified atypia as non-specific (AUCUS) and suspicious for malignancy (AUCH). In their report, more than 50% of patients with a positive biopsy had previous cytology reported as AUCH/Positive, and more than 80% as AUCUS/AUCH/Positive [8]. Based on studies looking for diagnosis agreement between cytology and biopsy, other authors consider that an AUCH diagnosis offers more sensitivity than a positive result in voided urine [9]. Bostwick et al. [10] do not agree that the subdivision of atypia (AUCUS and AUCH) results in an increase in sensitivity to carcinoma.
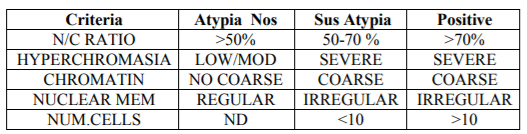
The Paris System (TPS) attempts to standardize the nomenclature and criteria for reporting urinary cytology. TPS focuses on the diagnosis of high-grade tumors, considering them to be more aggressive tumors that can compromise patient survival in a short period. TPS criteria are shown in Table I and are used to classify atypia as unspecific (AUC), suspicious (AUCH), or positive [7], establishing specific criteria for AUC (N/C ratio > 50% plus nuclear hyperchromasia) and AUCH (adding the presence of irregular nuclear membrane or coarse chromatin to AUC criteria). Barkan et al. [11] developed a diagnostic algorithm (Scheme 1) based on TPS. In this regard, they proposed a N/C ratio of over 50% accompanied by hyperchromasia or coarse chromatin or irregular membrane nuclei for AUC diagnosis. For AUCH diagnosis, an N/C ratio of more than 50% is needed, together with hyperchromasia and coarse chromatin or nuclear membrane irregularities. In AUCH cases, when 10 or more atypical cells are present in the smear, the diagnosis of positive for malignant cells must be made [11]. In another study [12], the authors observed an increase in the diagnosis of atypia when TPS was used, thereby increasing the false positive rate.
Here we applied TPS criteria to a retrospective series in order to evaluate the sensitivity and specificity using hierarchical criteria or using the number of criteria.
2. Material And Methods
We selected all the cases of urine cytology diagnosed as atypia during 2012 and 2013 from the case files of the Department of Pathology of the Hospital del Mar. We used the follow-up of the patients with cytological and or histological data, using the biopsy as a gold standard.
Three samples from three consecutive days were studied for all the patients. The samples were received without fixative liquid and were immediately centrifuged. The pellet was transferred to a Thin Prep test vial for fixing and processing. The slides were obtained using a T-5000 (Hologic, Malborough, Mass) and stained with an automated stainer (Leica Byosistems, Wetzlar, Germany). All the slides were submitted to automated lecture using the Imager (Hologic). As performed in gynecological cytology, we reviewed all the fields selected by the Imager (Hologic…) and, in the case of negative cases, we reviewed all the fields of the vertical diameter of the slide. In suspicious or positive cases, the whole slide was reviewed manually by an expert cytotechnologist and reviewed by a cytopathologist.
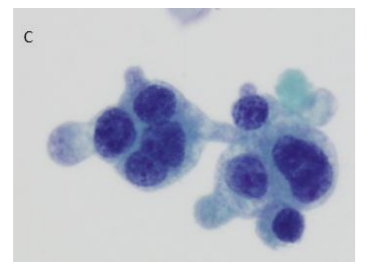
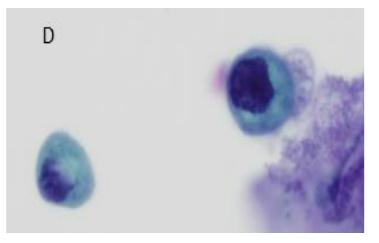
The criteria applied in this study are described in Table 1. They allowed the sub classification of cases as AUC, AUCH or Positive. For practical purposes, the N/R ratio was deemed to denote Positive when the N/C ratio exceeded 50%. In this case we did not distinguish whether the N/C ratio was above or below 70%. All the other criteria were evaluated as described in the table (Figure. 1). Furthermore, the following were evaluated: a) Atypical isolated cells; B) cytoplasmic vacuolization; c) central or peripheral nuclei; d) nuclear pleomorphism; e) presence of nucleolus; and F) thick nuclear membrane[6]. The statistical study was performed using a SPSS version 19 using a p < 0.005.
3. Results
A total of 123 cases were selected corresponding to 80 males and 53 females with a mean age of 69 (43-87).
The follow-up included 63 negative cases (51.2%) followed with cytology without a biopsy; 8 cases with atypia (6.5%) followed with cytology without a biopsy, and 52 cases (42.3%) with transitional cell carcinoma, all diagnosed by biopsy. Of the latter, 43 had high-grade urothelial carcinomas (Grade II and III), and 9 low-grade (Grade I).
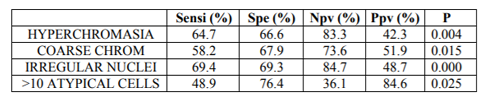
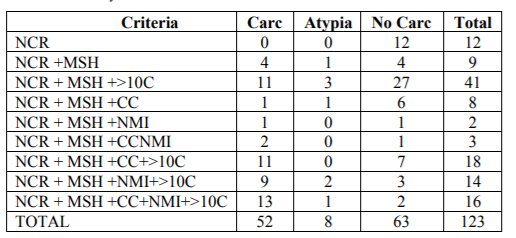
Regarding the cytological criteria, all the cases showed an N/C ratio over 50%; 111/123 cases (90.2%) showed moderate or severe hyperchromasia; 45/123 cases (36.6%) showed coarse chromatin; 35/123 cases (28.5%) showed nuclear membrane irregularities, and 89/123 cases (72.4%) showed more than 10 atypical cells. Hyperchromasia (p=0.004), coarse chromatin (p=0.015), nuclear membrane irregularities (p=0.000), and more than 10 atypical cells (p=0.025) showed statistical significance related to cancer in the follow-up Table 3 shows the number of cases with distinct cytological changes and the relationship with follow-up.
We looked for the correlation with follow-up in two ways.
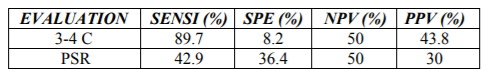
a) Considering the amount of cytological changes, including the number of atypical cells, we compared those cases with only 2 changes with those with 3 or four. The cases showing only one cytological change [12] and those 5 were excluded. Cases with atypia in the follow-up were also excluded from the analysis. None of the cases with 1 cytological criteria had cancer detected in the follow-up. 81.3% (13/16) cases with five criteria developed cancer in the follow-up. A total of 88 cases were studied. Of these, 8 had 2 cytological changes and 80 showed 3 or 4. The statistical values of 3-4 criteria for cancer in the follow up were: sensitivity 89.7% Specificity 8.2; Negative predictive value: 50%; Positive predictive value: 43.8% (Table 4).
b) TPS criteria application: cases with 3-4 criteria, excluding those showing more than 10 atypical cells were considered. A total of 18 cases were evaluated, 10 fulfilling 3-4 criteria and 8 fulfilling two criteria. Seven cases developed cancer and 11 were negative for cancer in the follow-up The sensitivity for cancer was 42.9%, the specificity 36.4%, the negative predictive value 50%, and the positive predictive value 30%
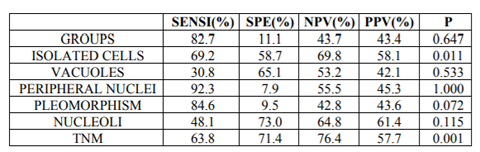
The following criteria—not considered in TPS—were evaluated in this study: a) single atypical isolated. b) cytoplasmic vacuolization; c) central or peripheral nuclei; d) nuclear pleomorphism; e) nucleoli; and F) thickness of nuclear membrane [6]. Of these, isolated cells (p=0.011) and nuclear membrane thickness (p=0.001) were statistically significant for cancer (Table 5).
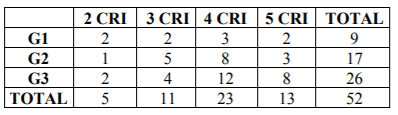
Table 6 shows the correlation between the number of criteria fulfilled and the tumor grade in biopsy. Most cancer cases fulfilled 3 or more criteria in the cytology, reaching 80.8% of Grade III carcinomas. Most cases with Grade I and II carcinoma did not fulfill 4 criteria and only 57.7% of Grade III carcinomas fulfilled four.
4. Discussion
Here we evaluated TPS criteria, sub-classifying the cases on the basis of number of criteria fulfilled independently of the quality of criteria, or following TPS classification. We excluded those cases fulfilling only 1 criterion since according to the recommendations of TPS, these cases must not be reported as atypia. All these cases were negative in the follow up. We also excluded those cases fulfilling all 5 criteria as they should be considered positive. In fact, only 13 of the 16 cases were confirmed positive in the follow up.
The sensitivity for cancer, based on the number of cytological changes, was higher (89.7%) than that achieved using TPS criteria (42.9%), but the specificity was lower.
We propose that TPS criteria should be used in a numerical manner, that is to say when a given case fulfills 3 or 4 criteria an AUCH should be diagnosed while in case only 2 criteria are observed the diagnosis would be AUC. In support of this proposal, none of TPS criteria showed sensitivity for cancer over 70% (Table II). Our results contrast with those reported in the literature [9,12,13].
Some aspects that can explain these differences are discussed. The first is the remarkable number of cases with 10 or more atypical cells. This observation could be attributed to processing with the Thin Prep Platform. No differences have been reported with cytosine use[14]. On the other hand, there is no established criterion regarding the minimum of number of urothelial cells for considering a sample sufficient for diagnosis. The criteria for considering a sample valuable focus on the amount of urine and on preservation of urothelial cells, but not the number of cells [7].
Also of note was the number of cases with moderate/severe hyperchromasia. This may be due to the use of automated reading (Imager). The use of the imager calls for the control of stain (in time and in volume of staining liquid related to the number of slides stained) in order to maintain the sensitivity of the apparatus. Thus, it is probably that this hematoxylin stains darker the smears than conventional. The use of an automated Imager system facilitates the workflow of the cytotechnicians and focuses the attention of the observer on specific cells and fields, excluding those fields without potential pathological cells.
Regarding the cytological diagnosis of Grade, I urothelial carcinoma, the problem remains unsolved. TPS is focused on the diagnosis of high-grade tumors and is not useful for diagnosis of low-grade ones. Furthermore, the criteria not included in TPS, such as isolated cells and nuclear membrane thickness, were observed in a half of the cytology of Grade I carcinomas.
We conclude that in our retrospective series TPS criteria were useful if a numerical approach is considered for diagnosing high-grade urothelial carcinoma but not for the diagnosis of low-grade carcinoma.
References
- Xie Q, Huang Z, Zhu Z, Zheng X, Liu J, Zhang M, Zhang Y. Diagnostic value of urine cytology in Bladder Cancer. A Meta-analysis. Ann Quant Cytopathol Histopathol 2016 Feb; 38(1): 38-44
- Zhang ML, Rosenthal DL, VanderBussche CJ. The cytomorphological features of Low-Grade Urothelial neoplasms vary by specimen type. Cancer Cytopathol 2016; 124: 552-64
- McCroskey Z, Pambuccian SE, Kleitherms S, Antic T, Cohen MB, Barkan GA, Wojcik EM. Accuracy and interobserver variability on the cytologic diagnosis of low-grade urothelial carcinoma in instrumented urinary tract cytology specimens. Am J Clin Pathol 2015 Dec; 144(6): 902-8
- Jackson J, Barkan GA, Kapur U, Wojcik EM. Cytologic and cystoscopic predictors of recurrence and progression in patients with low-grade urothelial carcinoma. Cancer Cytopathol 2013; 121 (7): 398-402
- Muus Ubago J, Metha V, Wojcik EM, Barkan GA. Evaluation of Atypical urine progression to malignancy. Cancer Cytopathol 2013; 171(7): 387-91
- Primary Tumors of Urinary Tract. Cap 6, pag 137 en Practical Urological Cytopathology. R.H.Bardales MD editor. Oxford University Press 2002
- Rosenthal DL, Wojcik EM, Kurtycz DFI editors. The Paris System for reporting urinary cytology. Springer. Heidelberg, Neew York, Dordrecht, London 2016.
- Rosenthal DL, Vandenbussche CJ, Burroughs FH, Sathiyamoorthy S, Guan H, Owen C, The Jons Hopkins Hospiutal template for urologic cytology samples: Cancer Cytopathol 2013; 121: 15-20
- Ton Un T, Kassouf W, Ahmadi-Kalifi B, Charbonneau M, Angar M, Bruno C, The value of suspicious for urothelial carcinoma cytology cathegory: A correlative study of 4 years including 337 patients. Cancer Cytopathol 2014. DOI: 10.1002
- Bostwick DG, Hossain D. Does subdivision of the atypical urine cytology increase predictive accuracy for orothelial carcinoma?. Diagn Cytopathol 2014; May. 17 DOI: 10.1002/dc23159
- Barkan GA, Wojcik EM, Nayar R, Savic-Prince S, Queck ML, Kurtycz DFI, Rosenthal DL. The Paris System reporting urinary cytology: The quest to develop a standardized terminology. Acta Cytologica 2016; 60: 185-197
- Granados R, Duarte JA, Corrales T, Camarmo E, Bajo P. Applying the Paris System for reporting urine cytology increases the rate of atypical urothelial cells in bening cases. A need for patient management recommendations. Acta Cytol 2017; 61: 71-76
- Joudi AM, Pambuccian SE, Wojcik EM, Barkan GA. The positive predictive value of “Suspicious for high-grade urothelial carcinoma” in urinary tract cytology specimens: A Single-Institution study of 665 cases. Cancer Cytopathol 2016 August 10. doi:10.1002/cncy.21764. [Epub ahead of print]Straccia P, Bizzarro T, Fadda G, Pierconti F.
- Comparison between cytospin and liquid based cytology in urine specimens classified according the Paris System for reporting Urinary Cytology. Cancer Cytopathol 2016 Jul; 124(7): 519-.23