Information
Journal Policies
ARC Journal of Nephrology
Volume-1 Issue-1, 2016, Page No: 5-10
Intradermal Hepatitis B Vaccine in Hemodialysis Patients Previously Non-Responsive To Intramuscular Hepatitis B Vaccine Route
Khalid Al Saran, Alaa Sabry, Mahmoud Ismail, Zakaria El Halwany, Nadera El Sayed, Nevein Farouk
Prince Salman center for Kidney Diseases, Riyadh, KSA
Citation : Al Saran K, Alaa S, Mahmoud I, Zakaria El H, Nadera El S, Nevein F. Intradermal Hepatitis B Vaccine in Hemodialysis Patients Previously Non-Responsive To Intramuscular Hepatitis B Vaccine Route. ARC Journal of Nephrology. 2016;1(1):5–10.
Copyright : © 2016 Al Saran K. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The aim of this study: Is to determine the response to intradermal hepatitis B virus (HBV) vaccination in individuals on hemodialysis (HD) whom were previously non responders to two or more courses of intramuscular hepatitis B virus (HBV) vaccination.
Methods: This prospective study included 15 patients (5 males and 10 females) patients on regular HD who previously received at least two courses of intramuscular hepatitis B vaccination and were non responsive. The study, started on May 2011 by intradermal injection of 10 µg Engerix hepatitis B vaccine above deltoid muscle weekly for eight weeks and after two months from last dose, the level of hepatitis B surface antibodies was checked two and six months later. According the result patients will be divided into two groups responder group (> 10 IU/L) and non- responders group (<10 IU/L). The study look at the factors which may affect the responsiveness to hepatitis B vaccination like gender, age, the number of HBV vaccine courses, the duration (years) since starting dialysis until starting the study, presence or absence of hepatitis c virus (HCV) infection, dialysis adequacy (evaluated by Kt/v), the length of hemodialysis session, hemoglobin level, albumin level, parathormone hormone level (pth), serum calcium, serum phosphorus levels and the original renal diseases.
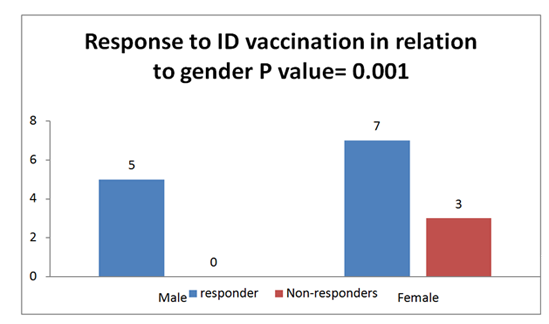
Results: 12 patients were responder ( 80.0% of the total patients number), the non-responders group include 3female patients (20.0% of the patients). The mean age in both the responder and the non-responder group were (56.25±13.71) and (71.33±5.03) years respectively (P value =0.09), HCV status has no effect on responsiveness HCV–ve patients total number eleven (responder 9 and nonresponder 2 ), HCV +ve patients total number four (responder 3 and nonresponder 1) the (P value=0.63), the mean number of HBV vaccine courses previously taken in responders and non responders group were (2.67± 0.98 and 2.67±0.58) respectively (P value=0.69). The mean of HD duration (years) in responders and non responders group were ( 4±1.65 vs 3.33± 1.52) respectively with (P value =o.55), the mean hemodialysis session length (hours) in responders and non responders group were (3.29± 0.26 vs 3.17± 0.29) respectively with ( P value = 0.54), the effect of nutritional status, (serum albumin as indicator) has no significance as the mean in responders and non responders group were ( 35.67± 4.7 vs 33.48±2.16 gm/dl) respectively (P value=0.27). The efficiency of dialysis on responsiveness using kt/v as indicator, the result in non responders higher than responders but without significant importance as shown in the mean and p value of both groups (1.55± 0.13 vs1.37±0.21) respectively ( P value=0.22).The hemoglobin, parathormone hormone, calcium and phosphorus were comparable in both group. More patients were responders compared to female patients with (P value =0.001).
Conclusion: We report a high response rate (80%) for intradermal HBV vaccination in non responder to intramuscular route of vaccination. We also found the female gender is the only factor, which decrease the response to intradermal HBV vaccine in Saudi HD patient.
Keywords: HBV vaccination, hemodialysis, intradermal, non-responder, Saudi.
1.Introduction
Patients on HD have a well documented impaired immune response to hepatitis B vaccination. These patients have lower seroconversion rates and faster declining titers of hepatitis B virus (HBV) antibodies than individuals who do not have end-stage renal disease; these patients might be at risk of viral and non viral infections due to immune compromised state[1]. The implementation of infection control strategies, the screening of blood donors for hepatitis B virus surface antigen (HBsAg) and antibodies against hepatitis B virus core antigen (anti-HBc) and the use of erythropoietin with the resultant decrease in blood transfusion requirements have resulted in a decreased incidence of hepatitis B virus infection in the HD population from 3% in 1976 to a stable incidence of 0.2% between 1987 and 1991[2]. Low or nonresponse to hepatitis B vaccination is seen in only a small proportion of people vaccinated with an adequate schedule and has a strong genetic basis[3]. The rate of low or nonresponse to hepatitis B vaccine is much higher in patients with uremia, also if co- infection with hepatitis C seems to further lower the response rate in such patients[4]. Approximately 90-95% of healthy people and 45-50% of dialysis patients properly respond to vaccination [5]. To increase efficacy of vaccination in dialysis patients, different methods of vaccination such as high dose of intra-muscular, subdermal, intradermal and adding adjuvant as erythropoietin or interleukin have been administrated[6] . To enhance the development of anti-HBs in HD patients by the intradermal (id) administration of HBV vaccine was reported to result in 100% antibody production in previously nonimmunized patients[7]. The rationale for using the intradermal route of vaccine administration is that a higher concentration of resident and recruited antigen- presenting cells is present within skin –associated lymphoid tissue than in striated muscle; this high concentration facilitates rapid trafficking of these activated cells and subsequent T-cell activation, which in turn, induces adaptive immune response8. Small studies and meta-analysis have suggested that intradermal vaccination for HBV is safe and effective in patients on hemodialysis9. This prompted us to evaluate the effectiveness of intradermal recombinant HBV vaccine in selected chronic HD Saudi patients previously non-responsive to intramuscular vaccination.
2.Patients and Methods
This prospective study was conducted in prince salman center for kidney diseases (PSCKD), Riyadh, KSA over a period of one year from May 2011 until May 2012 . This study include 15 patients (5 male and 10 females ), their age ranged from 26-82 year, all the subjects were negative for all serological markers of HBV infection, including HBsAg and anti-HBc antibodies also all negative for anti-HBs . We evaluated levels of HBs antibody (anti-HBs) titer two months after eight doses of intradermal vaccination; Engerix B, 10µg administered by intradermal injection over the deltoid muscle every two weeks. The range of dialysis session duration was from 3 to 4 hour, three times weekly dialysis schedule, with a blood flow rate range from 250 to 400 ml/min, the dialysate flow rate range from 500 to 800 ml/min and bicarbonate dialysis prescription was performed for all patients .Dialysis adequacy was assessed monthly calculation of by monthly calculation Single pool Kt/v (spKt/V), was assessed using the Daugirdas second-generation formula. Parathyroid hormone level (PTH), anemia by measuring the hemoglobin (Hb), calcium and phosphorus, nutritional state of the patients as determined by serum albumin. The previous numbers of intramuscular HBV vaccine courses, the original disease of chronic renal failure and the duration of hemodialysis per years were calculated.The factors which can affect the response to vaccine.
The results were summarized as the mean ± standard deviation (SD). Unpaired student’s t test for testing the significance of differences of values measured between responders and non responders was used, P value < 0.05 was taken as statistically significant. All analysis were performed using the SPSS version 16.The mann-Whitney rank-sum test was used as appropriate.
3.Results
A total of 15 patients were included in this study who were non responder to HBV vaccine after at least two courses of intramuscular HBV vaccine with titer level less than 10IU/L. They were vaccinated by10ug Engerix HBV vaccine weekly for 8 weeks by intradermal route. HBsAg was measured two and six months after the last dose.
Twelve out of fifteen patients (80%) were responder (HBsAb titre>10Iu/L), five of them have titer > 100Iu. This number increased to seven patients by repeated measurement 6 months later.
A total of 15 chronic HD patients non-responder to two courses of intramuscular HBV vaccine at PSCKD were enrolled in our study (5 males and 10 females). The mean age was 59.26±13.08 years (range26–82 y).The mean age was lower in the responder compared to the non-responder group (56.25±13.718 vs71.33±5.03 years respectively, P value=0.26). The etiology of end- stage renal failure were diabetes mellitus in 4 cases (26.6%), hypertension in 6 cases (40.0% ), glomerulo-nephritis one case (6.7%), congenital cause one case (6.7%) and unknown causes in 3 cases (20%).
Seven females out of ten while all males were responders (p value=0.001), see table 1 and figer1.No statistically significant difference was found between responder and non responder regarding the number of IM courses (2.67± 0.98 vs 2.67± 0.58 p value= 1.0).
Again no statistically significant difference was observed regarding dialysis duration between responder and non responder groups (3.29± 0.26 vs 3.17± 0.29 p value= 0.07), dialysis adequacy ( kt/v) (1.62± 0.46 vs 1.45± 0.07 p value= 0.13 , years on HD (4.0± 1.65 vs 3.33±1.53 p value= 0.55), ) for all patients were within the target without any difference between responder and non responder groups,Regarding nutritional state no significant difference between responder and non responder groups(serum albumin) ( 35.67± 4.71 vs 33.48± 2.17 with p value= 0.45).
Regarding bone profile parathormone hormone (the mean 52.81±18.19 p value=0.84), serum calcium (2.21±0.15 vs 2.26±0.04 p value=0.61),serum phosphorus (1.62±0.46 vs 1.45±0.07 p value=0.13), without significant difference between responder and non responder groups.
No statistically significant difference was found between responder and nonresponder regarding hemoglobin as indicator of anemia (11.20± 0.93 vs 11.77± 0.07 with p value= 0.32).
There was no significant difference between hepatitis C negative and positive patients regarding to responsiveness to ID HBV vaccine with (p value=0.64).
4.Discussion
The present study is undertaken with the aim of evaluating the efficacy of intradermal hepatitis B vaccine in nonresponder patients to IM route [10].There are two reasons for choosing the ID route of administration, firstly, it is less expensive and secondly, the skin is known to have a large numbers of antigen presenting cells, which enhance the immune response to vaccine [11]. This study result is in agreement with Barraclough etal [12]., and Chanchairuijira et al[13]. they were found revaccination by ID route, in stable HD patients previously not responder to IM vaccination had higher conversion rate, also rapid induction of protective level of antibodies. But the conflicted result were prescribed by Met et al[9]., and Sorkhi et al[14]. found that less seroconversion in patients vaccinated by ID and subcutaneous (SC) versus IM route.
Fabrezi et al.[15] Found no relation between age and seroconversion rate, this result in agreement with this study; which conflict with Chin16and Nancy M et al.[17] In their study found that the older age was being associated with a decrease responsiveness to HB vaccine.
Peces et al.[18]; and Navarrow et al[19]. Did not report any difference in response rate regard to duration of hemodialysis, this result in agree with our study result. This conflict the result of Steketee,R et al.[20] whom were observed that antibody response rates increases with increasing length of time on dialysis prior to receipt of vaccine but duration of dialysis has no association. Kovacic et al[21].and Salwa I. et al.26 Show that HBV vaccination response is weaker in hemodialysis patients with inefficient dialysis; this conflict our study and Nancy M et al.[17] Regina H. etal22. Low hemoglobin levels had no effect on response to HBV vaccine response, this result in agreement with this study and conflict with the result of McMaster KR et al [23] and Vlassopoulos D.5 Found low hemoglobin levels may have weaker immunologic response to vaccines. Peces et al[18] and Navarro etal[19] .not report any difference in the response rate with regard to serum albumin, this result in agreement with the result of our study, in contrast Fernandez et al.[24] had shown that malnutrition negatively influences the response to the HBV vaccine in hemodialysis patients; patients with serum albumin levels between 3-3.5g/dl were nonresponders in higher percentage than those with serum albumin levels between 4.5-5g/dl. Nancy M.et al[17] found that primary origin of the ESRD had no statistically significant effect on response to HBV vaccine, this in agreement with our study result, conflicting the study result of Fabrizi F. et al[27] who found that diabetes mellitus (DM), an important cause of unresponsiveness to HBV vaccine.
Regarding to HCV positive patients, we not found any effect on responsiveness to HBV vaccine, this result in agreement with result of Urbanowicz W.[25] and Salwa I.et al[26], but conflict the result of Navarro et al[4].He reported a low response to HBV vaccination in HCV-infected hemodialysis patients.
Salwa I.et al.[26] and DaRoza G. et al.[28] found parathyroid hormone level did not significantly influence antibody response to hepatitis B immunization, their result in agreement with our study result.
Nahar et al.[29] and Sezer et al[30].found antibody response rate was higher in female than male subjects and it was highly statistically significant, this conflict the result in our present study where the male response is highly significant than female. Peces et al[18]. and Marangi et al.[30] studies have observed that the subjectʼs gender did not influence the response rate to hepatitis B vaccine in hemodialysis patients.
5.Conclusion
We reported a higher response rate to intradermal hepatitis-B vaccination in previously non responder to at least two courses of intramuscular vaccination. The gender was the only factor affect the response to hepatitis B vaccine, other factors such as age, efficiency of dialysis, the cause of ESRD, anemia, HCV +ve antibodies status ,the number of HBV vaccine courses, the duration of dialysis, the length of hemodialysis session and parathormone hormone level, calcium and phosphorus had no association with response to ID HBV vaccination route.
We suggest the administration of low –dose intradermal inoculation in order to re-vaccinate dialysis individuals showing unresponsiveness to HB vaccine.
6.References
- Vanholder R., Van Loo A., Dohndt A. M.et al. Influence of uremia and hemodialysis on host defense and infection. Nephrol Dial. Transplant 1996;11: 593-598.
- Tokars JI, Alter MJ, Favero MS, Moyer LA and Bland LA: National surveillance of dialysis –associated diseases in the United States,1991. ASAIO J 1993;39: 966-975.
- Höhler T, Reuss E, Evers N et al. Differential genetic determination of immune responsiveness to hepatitis B surface antigen and hepatitis A virus: A vaccination study in twins. Lancet 2002; 360: 991-995.
- Navarro JF, Teruel JL, Mateos ML and Ortuño J. Antibody level after hepatitis B vaccination in hemodialysis patients: Influence of hepatitis C virus infection. Am J Nephrol 1996:16:95-97.
- Vlassopoulos D. Recombinant hepatitis B vaccination in renal failure patients. Curr Pharm Biotechnol 2003;4(2):141-51.
- Kayatas M. Levamisole treatment enhances protective antibody response to hepatitis B vaccination in hemodialysis patients. Artif Organs 2002;26(6): 492-6
- Ono K and Kashiwagi S: Complete seroconversion by low dose intradermal injection recombinant hepatitis B vaccine in hemodialysis patients. Nephron 1991;58:47.
- BarracloughK.A. et.al. Intradermal versus intramuscular hepatitis B vaccination in hemodialysis patients: a prospective open-label randomized controlled trial in nonresponders to primary vaccination. Am. J.Kidney Dis 2009 ; 54: 95-103.
- Mat.O., Mestrez F., Beauwens R. et al. Primary high dose intradermal hepatitis B vaccination in hemodialysis : cost-effectiveness evaluation at 2years. Hemodial.int. 2006; 10: 49-55.
- Rajadhyaksha KP, Haridas V, Plunber ST et al.Efficacy of low dose intradermal hepatitis B. IndJ Gastroenterol 1988: 7: 111.
- Wahl M, Hermodsson S. Intradermal, subcutaneous or intramuscular administration of hepatitis B vaccine: side effects and antibody response. Scand J Infect Dis 1987;19(6):617-21.
- Barraclough KA,WigginsKJ, Hawley CM. Intradermal versus intramuscular hepatitis B vaccination in hemodialysis patients: a prospective open- label randomized controlled trail in nonresponders to primary vaccination. Am J Kidney Dis 2009; 54:95-103.
- Chanchairuijra T, Chantaphakul N, Thanwandee T et al. Efficacy of intradermal hepatitis B vaccination compared to intramuscular vaccination in hemodialysis patients. J Med Assoc Thai.2006;89:33-40.
- Sorkhi H, Dooki MR, and Ebrahimmnejad MS. low dose intradermal and subcutaneous versus intramuscular hepatitis B vaccination in primary non-responding hemodialysis patients. J Med Assoc Thai 2006: 89: 1648-1653.
- Fabrizi F, Martin P, Dixit V et al. Meta-analysis :the effect of age on immunological response to hepatitis B vaccine in end-stage renal disease. Aliment Pharmacol Ther 2004;20: 1053- 1062.
- Chin Al. Hepatitis B virus vaccine response in hemodialysis: baseline patient characteristics. Kidney international, 2003;4: 296-303.
- Nancy M, Lisa G and Marc B. Successful Vaccination With Intradermal Hepatitis B Vaccine in Hemodialysis Patients Previously Nonresponsive to Intramuscular Hepatitis B vaccine. J. Am. Soc. Nephrol. 1995; 5: 1930-1934.
- Peces R, Torre M, Alchzar R and Urra JM. Prospective analysis of the factors Influencing the antibody response to hepatitis B vaccine in hemodialysis patients. American Journal of Kidney Diseases.1997; 29: 239-245.
- Navarro JF, Teruel JL, Mateos M, Ortuno J. Hepatitis C virus infection decrease the effective antibody response to hepatitis Bvaccine in hemodialysis patients. Clin Nephrol. 1994; 41 : 113- 116.
- Steketee, R. W., Ziarnik, M.E., and Davis, J.P. Seroresponse to hepatitis B vaccine in patients and staff of renal dialysis centers. Am J Eptoemlol 1988;127: 772-82.
- Kovacic V, Saint M, Vukman V. Does efficient haemodialysis improve the response to hepatitis B virus vaccination? Lijec Vjesn. 2004;126:133-7.
- Regina H.Medeiros; Ana Elizabeth PL Figueiredo; Carlos Eduardopolide-Figueiredo; Domingos Otávio d´Avila; Carlos Abaeté de los Santos. Low response to intradermal hepatitis B vaccination in incident hemodialysis patients. J. Bras.Nefrol. 2011;33:576-582.
- McMaster KR 3rd, Roper JK, Carter JB. Intradermal hepatitis B vaccination in a 300-bed primary care hospital: experience with a recombinant vaccine in a four dose schedule. Am J Infect Control 1993;21:283-8.
- Fernanadez E, Betriu MA and Gomez R. Response to the hepatitis B virus vaccine in hemodialysis patients: influence of malnutrition and its importance as a risk factor for morbidity and mortality. Nephrol Dial Transplan.1996; 11: 1559-1563.
- Urbanowicz W. Efficacy of prophylactic vaccination against hepatitis B virus infection in hemodialyzed patients. Prezegl Epidemol.2000;54: 343-350.
- Salwa Ibrahim, Sharaf El Din, and Ibrahim Bazzal. Antibody level after Hepatitis–B Vaccination in hemodialysis Patients: Impact of Dialysis Adequacy, Chronic Inflammation, Local Endemicity and Nutritional Status. J. Of The National Medical Association. 2006;98:1953-1957.
- Fabrizi F, Di Filippo S, and Marcelli D. Recombinant hepatitis B vaccine use in chronic hemodialysis patients: Long-term evaluation and cost-effectiveness analysis. Nephron 1996;72:536-543.
- DoRoza G, Loewen A, and Djurdjev O. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am JKidney Dis.2003; 42: 1184-1192.
- Nahar K., Jahan M., Nessa A,and Tabassum S. Antibody responses after hepatitis B vaccination among maintenance hemodialysis patients. Bangladish Med Res Counc Bull 2011; 37: 88-91.
- Sezer S, Ozdemir F.N., Guz G et al. Factors influencing response to hepatitis B virus vaccination in hemodialysis patients.Transplantation proceedings 2000; 32: 607-608. 24. 31.Marangi AL, Giordano R and Montanaro A. Hepatitis B virus infection in chronic uremia: Longterm follow-up of a two-step integrated protocol of va-cination. Am J Kidney Dis 1994; 23 : 537-542