Information
Journal Policies
Hydrolytic and Degradative Activities of Zn2+ Ions for Bacterial Cell Walls, Viral RNA Degradation, and Regulation of Tumorous Cell Growth
Dr. Sci. Tsuneo Ishida
Copyright : © 2018 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Zn2+ ions-associated hydrolytic and degradative activities (ZnAHDAs) for bacteria are due to bacteriolyses and destructions of bacterial cell walls by the inhibitions of peptidoglycan (PGN) elongation owing to activated PGN autolysins. ZnAHDAs for viruses are viral protein hydrolysis, viral mRNA degradation, and bacteriophage viral-endolysins. Especially, the striking characteristics for viral hydrolyzing and degrading activities are virus-induced mRNA degradation and bacteriophage-viral endolysins that these Zn2+ induced enzymes lead to viral apoptotic deaths. Furthermore, ZnAHDAs for cancer cell are proteasome and autophage that lead to cancer and tumor cell death, Zn2+ induced Zrt-, Irt-like protein 1 (ZIP1) that inhibits malignant tumor and proliferation, and zinc complex and zinc chelation that have important roles for anticancer/tumor apoptotic death. Thus, these hydrolysis and degradation method is now noteworthy under the hydrolase enzymes development for bactericidal, virucidal, and cancerous cell deaths.
Bacteriolysis and destruction, PGN autolysin, Hydrolysis, Viral protein and RNA degradation, Bacteriophage, Malignant tumor, Zinc chelation,Immunology and Vaccines
1. Introduction
Sufficient availability of zinc is of particular importance to the immune system and vaccination. Zinc induced high immunology leads to vaccination that zinc could improve rotavirus vaccine (RV) immunologenicity by altering the intestinal microbiota and immune function [1]. Zinc ion solution is considered as vaccine solutions [2], containing ZnO nanoparticles solution [3] for virus vaccine. Zinc species of zinc sulfate, zinc acetate and zinc sulfate, and zinc sulfate for tetanus, cholera, and influenza diseases, respecti-vely, are used as zinc supplementation and vaccination with highly zinc-dependent functions of immune system [4]. However, the physiological effect on the immune system contributes significantly to the results observed in supplementation trial for different diseases, and in zinc excess, a balanced zinc homeostasis is crucial for either defending against invading pathogens or protecting the human body against an overreactive immune system causing auto-immune diseases, chronic inflammation or allergies [5]. Zinc is an essential trace element, and the human body has efficient mechanisms, both on systemic and cellular levels, to maintain homeostasis over a broad exposure range that the human body contains 2-3 g zinc which on the cellular level, 30-40% of zinc is localized in the nucleus, 50% in the cytosol and the remaining part is associated with membranes [6]. Apoptosis of zinc ions is accumulation of intracellular zinc, either as a consequence of exogenous administration or release from intracellular stores by reactive oxygen species (ROS), activates pro-apoptotic molecules like p38 and potassium channels, subsequently lead to apoptosis, necrosis, and cell death [6].
Immunological effects of zinc ions are an immune regulatory influence. Zinc is known to have systemic effects such as regulation of the immune system as well as direct cellular effects resulting in regulation of gene expression [7], bioenergetics, signal transduction and cell invasion. The zinc effects are involved in the regulation of apoptosis in malignant cells and the effects on cancer cells must be viewed from the perspective of physiological regulation for zinc homeostasis [8]. Zinc complexes as bactericide, anti-virus, anti-tumor agents are used such as zinc nitrate Zn(NO3)2, zinc sulfate ZnSO4, and zinc oxide ZnO nanoparticles (ZnO NPs) [9].
In this mini-review, in order to elucidate serious diseases, it is focused and discussed that Zn2+ ions promote bacteria-, viruses-, and tumors-associated hydrolyzing and degrading enzyme activations and the resulting to lead to bacterial, viral, and tumor cell death.
2. Zn2+ Ions Promote Bacteria-Associated Hydrolyzing And Degrading
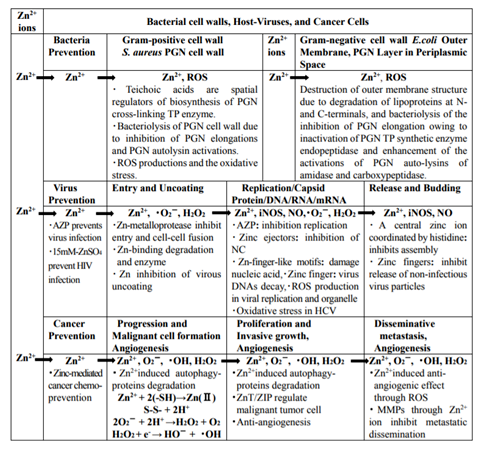
Bacteriolysis against S. aureus peptidoglycan (PGN) cell wall by Zn2+ ions is due to inhibition of PGN elongation caused by regulation of PGN synthetic transglycosylase (TG) and transpeptidase (TP), and enhancement of the activation of PGN autolysins of amidases [10]. The other, bacteriolysis and destruction against E. coli cell wall by Zn2+ions are caused by the destruction of outer membrane structure due to degradative enzymes of lipoproteins at N- and C-terminals, and by the inhibition of PGN elongation owing to inactivation of PGN TP synthetic enzyme endopeptidase and enhancement of the activations of PGN hydrolases and autolysins of amidase and carboxy-peptidase[10].
These characteristics is that the activated PGN autolysins are largely contributed for suppression and regulation of bacterial cell growth.
3. Zn2+ Ions Promote Virus-Associated Hydrolyzing, Degrading, And Endolysin; Virus Restriction Factor, Rna Degradation, And Host-Cell Defense
Virus restriction factors may be in presence of viral entry, viral DNA synthesis, intracellular movement of viral nucleic acids and viral gene expression. These restriction systems constitute newly appreciated components of an innate immunity that may be important for survival of a host exposed to virus infections which one of these restriction systems is selectively degradation of viral mRNA [11]. In general, RNA is degraded at the end of its useful life, which is long for a ribosomal RNA but very short for excised introns or spacer fragments that is closely regulated for most mRNA species. RNA molecules with defects in processing, folding, assembly with proteins are identified and rapidly degraded by the surveillance machinery [12]. Zinc finger antiviral protein (ZAP) specifically binds to the viral mRNA and recruits the cellular RNA degradation machinery to degrade the target RNA which for viruses to escape ZAP-specific viral mRNA degradation, one intriguing possibility is that viruses might encode factors that either inactivate ZAP or block ZAP-mediated RNA degradation [13]. The degradation is mediated by the viral RNA poly-merase that associates with host RNA polymerase Ⅱ (Pol Ⅱ ) that increased ubiquitylation of Pol Ⅱ in infected cells and upon the expression of the viral RNA polymerase suggesting that the proteasome pathway plays a role in Pol Ⅱ degradation [14]. ZAP also inhibits HIV-1 infection by promoting the degradation of specific viral mRNAs that overexpression of ZAP rendered cells resistant to HIV-1 infection in a ZAP expression level-dependent manner, whereas depletion of endogenous ZAP enhanced HIV-1 infection [15]. Thus, depletion of each of these mRNA degradation enzymes reduced ZAP’s activity and ZAP inhibits HIV-1 by recruiting both the 5’ and 3’ mRNA degradation machinery to specifically promote the degradation of multiply spliced HIV-1 mRNAs. Zinc mesoporphyrin (ZnMP) selectively and markedly down-regulated nonstructural 5A(NS5A) protein levels by increasing degra-dation of NS5A protein that ZnMP may hold promise as a novel agent to treat HCV infection [16]. Tripartite motif-containing protein 25 (TRIM25) also is required for the antiviral activity of ZAP that downregulation of endogenous TRIM25 abolished ZAP’s antiviral activity [17]. The TRIM25 is required for the antiviral activity of ZAP that down-regulation of endogenous TRIM25 remarkably abolished ZAP’s activity. TRIM25 is required for ZAP optimal binding to target mRNA. Several mammalian viruses encode factors that broadly dampen gene expression by directly targeting mRNA that these factors promote mRNA degradation to globally regulate both host and viral gene expression, in which in some cases, there is a lack of selectively for degradation of host versus viral mRNA, indicating that the purposes of virus-induced mRNA degradation extend beyond redirecting cellular resources towards viral gene expression [18]. In addition, several antiviral pathways use RNA degradation as a vital restriction mechanism, and these host-encoded ribonucleases target and destroy viral RNA [18]. RNA degradation in viral replication and antiviral defense leads to destroy viral RNA and restrict virus[19]. Virus-associated hydrolyzing and degrading may be applied to bacteriophage-viral endolysins.
As described-above, the striking characteristics for viral hydrolyzing and degrading activities are virus-induced mRNA degradation and bacteriophage-viral endolysins that these Zn2+ induced enzymes lead to viral apoptotic deaths.
4. Zn2+ Ions Promote Tumor-Associated Serine Hydrolyzing And Degrading
Normal cellular growth and development require a balance between protein synthesis and degradation. Eukaryotic cells have two major avenues for degradation: Proteasome and Autophagy [20]. Autophagy as self-eating is involved in the bulk degradation, in which autophagy is highly conserved homeostatic mechanism for the degradation and recycling of bulk cytoplasm, organelles, and long-lived proteins through the lysosomal machinery. While functional autophagy prevents tumor growth initiation, its pro-survival effect may allow transformed cells to resist against progression of diseases. Degradation of the mutant protein by Zn2+ ion mediated and induced autophagy lead to cell death in cancer cell line [21,22] and activation of NKG2D ligands in tumor immunity [23]. Further, autophagy in tumor immune micro-environment can affect immune responses inside the tumors. The autophagy in tumor cells play dual roles of immunoglobulins and immune-related cells in tumor development [24].
Zinc ions can significantly contribute to the progression of tumor disease and to the ability of prostate cell lines to metastasize, where several compounds in order to be zinc-presence in the cancer cells act as an inhibitor of apoptosis [25]. It prevents both apoptosis dependent on caspases and oxidative necrosis. Consequently, these effects of zinc also impose anti-tumor action, in which the ability of prostate cells to accumulate zinc is due to the expression and activity of the zinc uptake transporter, ZIP1 as a tumor suppressor gene in prostate cancer [26].
Zinc compounds have many biological activities, including the ability to induce apoptosis in cancer cells. Zinc oxide nanoparticles are attributed to vital role in cancer eradiation, that an important advantage of the targeted tumor treatment is lowering the cyto- and genotoxicity of active substance [27]. MMPs remain a viable target for cancer therapeutics. The role of MMPs in cancer, clinical trials for MMP inhibitors, and novel approaches to targeting MMPs in cancer [28]. Clioquinol targets NF-kB and lysosome pathways independently, favoring further development of clioquinol as a novel anticancer agent [28]. The p53 pathway of rapid advances has been developed in small molecule protein-protein interactions inhibitors, in which now increased understanding needed is p53 that is activated selects its response between reversible growth arrest apoptosis or senescence [29]. Zrt-, Irt-like protein (Zip) and zinc transporter (ZnT) or both zinc and metallothioneins (MTs) have important roles for anti-cancer activities of cancer and tumor cells [30]. These Zn compounds as multipurpose compounds, biological roles in homeostasis, proliferation and roles in immunity and in chronic diseases, such as cancer, brain tumor.
These important results obtained above are that Zn2+ ion-mediated hydrolyzing and degrading highly activity for anticancer is found to be by proteasome and autophagy that an important advantage of the target tumor treatment is lowering the genotoxicity of active substance. In addition, it makes use of a relatively new method termed activity-based proteomics to identity a protein with serine hydrolase activity that is an essential regulator of tumor cell growth and cell death which by using the functional approach, being able to identify a specific enzyme target that may serve as a valuable target for development of anticancer drugs.
As described above, hydrolytic and degradative activities of Zn2+ ions for bacterial cell walls, host-viruses, and cancer/tumor cells are summarized in Table 1.
5. Conclusion
For bacteria, Zn2+ ions promote activation of PGN-hydrolases and autolysins, and inhibition of PGN elongation, subsequently lead to bacteriolysis and destruction of bacterial cell walls. For viruses, depletion of each of these mRNA degradation enzymes reduced ZAP’s activity and ZAP inhibits HIV-1 by recruiting both the 5’ and 3’ mRNA degradation machinery to specifically promote the degradation of multiply spliced HIV-1 mRNAs. TRIM25 is required for the antiviral activity of ZAP that downregulation of endogenous TRIM25 abolished ZAP’s antiviral activity. RNA degradation in viral replication and antiviral defense leads to destroy viral RNA and restrict virus. In addition, virus-associated hydrolyzing and degrading may be applied to bacteriophage-viral endolysins.
Hydrolysis and degradation of cancer proteins by Zn2+ ions through autophagy, Zn-induced malignant tumor/ inhibition of proliferation, and zinc-complex and zinc chelation as anticancer high activity have an efficiency for the regulation of cancer and tumor cell growths.
In summary, Zn2+ ions-associated hydrolytic and degradative activities (ZnAHDAs) for bacteria are due to bacteriolyses and destructions of bacterial cell walls by the inhibitions of PGN elongation owing to activated PGN autolysins. ZnAHDAs for viruses are viral protein hydrolysis, viral mRNA degradation, and bacteriophage-viralendolysins. Furthermore, ZnAHDAs for cancer cell are proteasome and autophage that lead to cancer and tumor cell death, Zn2+ induced ZIP1 that inhibits malignant tumor and proliferation, and zinc complex and zinc chelation that have important roles for anticancer/tumor apoptotic death. Thus, these hydrolysis and degradation method is now noteworthy under the hydrolase enzymic development for bactericidal, virucidal, and cancerous cell deaths.
References
- R.P. Lazarus, J. John, E. Shanmugasundaram et al; The effect of probiotics and zinc supplementation on the immune response to oral rotavirus vaccine: A reandomized, factoria design, placebo-controlled study among Indian infants, Vaccine, 2018; 36:273-279.
- Abdulwahab MA Teimesani; Oral rehydration salts, zinc supplement and rota virus vaccine in the management of childhood acute diarrhea,
- N. Sharma, S. Jandaik and S. Kumar; Synergistic activity of doped zinc oxide nanoparticles with antibiotics: ciprofloxacin, ampicillin, fluconazole and amphotericin B against pathogenic microorganisms, Annals of the Brazilian Academy of Sciences, 2016; 88(3):1689-1698.
- Silke Overbeck, Lothar Rink and Hajo Haase; Modulating the immune response by oral zinc supplementation: a single approach for multiple diseases, Arch. Immunology Ther. Exp., 2008; 56:15-30.
- Inga Wessels, Martina Maywald, and Lothar Rink; Zinc as a gatekeeper of immune function, Nutrients, 2017; 9:1-44.
- L.M. Plum, L.Rink, and H.Haase; The essential toxin: Impact of zinc on human health, Int.J.Environ Res.Public Health, 2010; 7:1342-1365.
- Noga Dubi et al: Extracellular zinc and zinc-citrate, acting through a putative zinc-sensing receptor, regulate growth and survival of prostate cancer cells, Carcinogenesis, 2008; 29, Issue 9:1692-1700.
- Renty B.Franklin and Leslie C. Costello: The important role of the apoptotic effects of zinc in the development of cancers, J Cell Biochem. 2009 April 1; 206(5):750-757.
- S. Sabir, M. Arshad and S.K. Chaudhari; Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications, The Scientific World Journal, 2014; 2014:1-9.
- T. Ishida, Antibacterial Mechanism of Bacteriolyses of Bacterial Cell Walls by Zinc(Ⅱ) Ion Induced Activations of PGN Autolysins, and DNA damages, Journal of Genes and Proteins, 2017; Vol.1, Issue 1:1-7.
- Stephen P. Goff: Retrovirus restriction factors, Molecular Cell, 16(2004)849-859.
- Jonathan Houseley and David Tollervey: The many pathways of RNA degradation, Cell, 2009, 136: 763-776.
- Yiping Zhu and Guangxio Gao: ZAP-mediated mRNA degradation, RNA Biology, 5, issue, 2008, 2: 65-67.
- F.T.Vreede, A.Y. Chan, J.Sharps, E.Fodor: Mechanisms and functional implications of the degradation of host RNA polymerase Ⅱ in influenza virus infected cells, Virology, 2010,396: 125-134.
- Yiping Zhu, Guifang Chen, Fengxiang Lv, Xinlu Wang et al: Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation, PNAS, 108, No.38(2011)15834-15839.
- W.Hou, Q.Tian, J.Zheng, and H.L.Bonkovsky: Zinc mesoporphyrin induces rapid proteasomal degradation of hepatitis C nonstructural 5A protein in human hepatoma cells, Gastroenterology, 2010, 138: 1909-1919.
- Xiaojiao Zheng, Xinlu Wang, Fan Tu et al: TRIM25 is required for the antiviral activity of zinc finger antiviral protein, Journal of Virology, 2017; 91, issue 9:1-17.
- Emma Abernathy and Britt Glaunsinger: Emerging roles for RNA degradation in viral replication and antiviral defense, HHS Public Access, Virology, (2016)1-13.
- Emma Abernathy, Britt Gaunsinger: Emerging roles for RNA degradation in viral replication and antiviral defense, Virology, 2015; 479-480:600-608.
- Erica John et al: Zinc in innate and adaptive tumor immunity, J. Translational Medicine, 2010; 8:118-134.
- Jung Jin Hwang et al: Zinc(Ⅱ) ion mediates tamoxifen-induced autophagy and cell death in MCF-7 breast cancer cell line, Biometals, 2012; 23:997-1013.
- A Garufi et al: Degradation of mutant p53H175 protein by Zn(Ⅱ) through autophagy, Cell Death and Disease,2014; 5: 1-9.
- N Nausch and A Cerwenka: NKG2D ligands in tumor immunity, Oncogene, 2008; 27:5944-5958.
- Chia-Jung Li et al: New insights into the role of autophagy in tumor immune microenvironment, International Journal of Molecular Sciences, 2017; 18:1566-1580.
- MARKETA AZTALMACHOVA et al: Effect of zinc ions on the expression of pro- and anti-apoptotic factors in high-grade prostate carcinoma cells, ONCOLOGY REPORTS, 2012; 28:806-814.
- Renty B. Franklin and Leslie C.Costello: Zinc as an anti-tumor in prostate cancer and other cancers, Arch Biochem Biophys. 2007 July 15; 463(2):211-217.
- J.Bogdan, J. Plawinska-Czanak and J. Zarzynska: Nanoparticles of titanium and zinc oxides as novel agents in tumor treatment: a review, Nanoscale Research Letters, 2017; 12:225-240.
- H.Yu, J.R.Lou and W.Q.Ding: Clioquinol independently targets NF-kB and Lysosome pathways in human cancer cells, Anticancer Research, 2010; 30:2087-2092.
- D.P.Lane, C.F. Cheok, and S.Lain: p53-based cancer therapy, Cold Spring Harbor Perspectives in Biology, 2017 Oct 30:1-19.
- Christos T. Chasapis et al: Zinc and human health: an update, Arch Toxicol, 2012; 86:521-534.