Information
Journal Policies
MHC II (HLA-DR) on PMNs Stimulated with LPS in Autologous Culture of Total Human Leukocytes with Positive Serology for Chagas Disease
Fernando Marcos Rodriguez1,Rebeca Rinero1,Maria Victoria Reyna1,Adriana Haydee Vargas2,Claudia Leonor Carabajal Miotti2,Natalio Emilio Gonzalez Silva2,Susana GracielaRuiz de Frattari2,Ivon Teresa Clara Novak1*
2.Institute of Hematology and Hemotherapy, National University of Cordoba, Cordoba, Argentina.
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Activated humanT lymphocytes express Class II MHC in addition APCs. LPS stimulation can induce the expression of HLA-DR molecules in other cells and our objective was to observe their expression in PMNs in cellular interactions in culture of total leukocytes in samples with positive Chagas serology.
From healthy human blood samples (n = 10) and samples with positive serology for Chagas (n = 6) anticoagulated with Heparin; with ethical consent approved by ethical committee of National Hospital Clinicas, autologous leukocyte cultures were performed and in vitro stimulation with LPS, and controls without stimulation. Samples were performed with HLA-DR Immunofluorescence technique. Nuclear staining with DAPI. Some samples were processed for ultrastructural study of cell interactions with TEM.
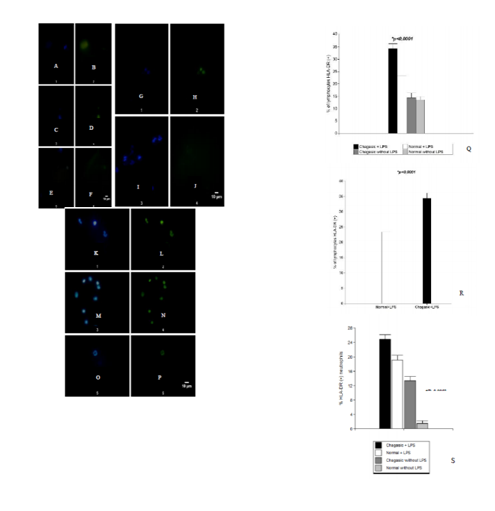
Results: LPS stimulation induced expression of HLA-DR molecules in PMNs in both samples and samples from positive Chagas serology showed a higher number of HLA-DR + lymphocytes and HLA-DR + PMNs (p < 0.001, t-test for paired samples). Cell-cell interactions between PMNs and lymphocytes were observed. Lymphocytes showed abundant cytoplasm, and polarization of mitochondria. Conclusions: The ultrastructural aspects reveal signs of cellular activation in chagasic samples and interactions between PMNs and lymphocytes. Expression of HLA-DR in PMNs could involve MHC ClassII antigen presentation pathway to CD4+ T lymphocytes.
HLA-DR, MHC Class II, PMN, leukocytes, Chagas,Immunology and Vaccines
1. Introduction
A fundamental step consisting of the presentation of the antigen to the T lymphocytes by antigen-presenting cells (APCs) initiate adaptive immune responses. The processing and presentation of antigens requires that the antigenic peptide / major histocompatibility complex (MHC) complexes are expressed on the cell surface in APCs. It involves the action of proteases that cleave the antigenic protein in small peptides, which are associated with Class I or Class II molecules of MHC[1,2]. Class I MHC molecules present peptides cytosolic to CD8 T lymphocytes and act as ligands of NK cell receptors. MHC Class II molecules present peptides by the endosomal pathway to CD4 T lymphocytes [2]. MHC Class I molecules are constitutively expressed in almost all tissues, at high levels in lymphoid cells, moderate in most cells and low in other cells (eg hepatocytes). Its expression can be increased by the effect of IFNα, β and γ [3]. MHC Class II molecules are constitutively expressed in: B lymphocytes, monocytes, dendritic cells, erythroid precursors,Langerhans cells, thymic epithelium, Kupffer cells and human activated T lymphocytes. Its expression can be induced in T lymphocytes, NK cells, endothelial cells, keratinocytes, melanocytes, astrocytes and fibroblasts by the action of IFNγ [3].Molecules required for antigen presentation and activation of T cells as MHC Class II are found in cytoplasmic reservoirs of human PMNs and are translocated after stimulation with PMN activators such as phorbol myristate acetate (PMA), lipopolysaccharide (LPS) or with peptides [4,5]. This finding may imply the possibility that PMNs have a role in the presentation of antigens to T lymphocytes.T cells only recognize antigens that appear on cell surfaces in the context of Class I or Class II MHC molecules [2].
Chagas disease is a parasitic disease produced by the protozoan Trypanosoma cruzi transmitted to humans by triatomines [6]. In Latin America Chagas disease affects more than 10 million people but it has become a global health problem due to migration [7,8]. Chronic cardiomyopathy is the main consequence of the infection, it developed in 30% of cases and immunological mechanisms are involved largely by the Th1 lymphocyte profile [7]. The pathogenesis of chronic Chagas cardiomyopathy is associated with parasite persistence, inadequate immune response and autoimmunity [9].
In chronic stage, neomycrovasculature has been described as an active factor in immunopathology [10]. In Chagas disease pathologic inflammatory infiltrate in cardiac tissue consists mainly of lymphocytes, but macrophages, plasma cells, eosinophils and neutrophils have also been observed [11].
We performed an study of the morphology characteristics and the behavior of polymorphonuclear neutrophils (PMNs)in healthy patients and with positive serology for Chagas disease [12]. In human neutrophils ring nucleus were observed earlier by Cabral in 1987 in chagasic patients and healthy individuals[13,14]. We report extracellular traps (ETs) formation in ring cells in culture of chagasic patients without lipopolysaccharide(LPS) stimulation. We observed increased longevity of polilobulated PMN in chagasic patients, after 72 hours of autologous culture, viable neutrophils with polilobulated nucleus persisted. The presence in the culture medium (added with filtered serum from the same donor) of cytokines and inflammatory mediators released by macrophages and lymphocytes in samples from patients may be prolong the half-life of PMNs. We reported increased number of ring cells and high positivity to myeloperoxidase (MPO) in chagasic patients. MPO housed in the azurophilic granules is part of the system of oxygen dependent cytotoxicity of PMNs. The presence of this enzyme in ring cells of healthy donors, as well as those of chagasic donors is highly positive comparable to that observed in polilobulated neutrophils in both groups. This high positivity could be related to a high increase in MPO activity to microbicidal function [15]. In other work we observed expression of costimulatory molecule CD80 in neutrophils in samples with positive serology for Chagas and it would induce an immunoregulation. The presence of costimulatory molecules could be related to the possibility of rupture of self-tolerance in syntony with autoimmune phenomena described in Chagas’ disease[16].
In previous work we found CD80 and CD86 colocalized in neutrophil extracellular traps (NETs) in autologous leukocytes cultures, after LPS or ovalbumin (OVA) stimulation. The presence of CD80 and CD86 in NETs could influence the cell environment through the B7-1/B7-2:CD28/CTLA-4 pathway. The importance of this finding resides in neutrophils functions, the possibility of acquire competence to be APC and playing a role in immunomodulation[17]. T lymphocytes respond innately to LPS with signaling via the TLR4 receptor. LPS does not affect the proliferation or cytokine secretion of T lymphocytes but increases adherence and inhibits their migration [18]. LPS stimulation can induce the expression of HLA-DR molecules (MHC Class II) in other cells besides APCs. In the present work our objective was to observe HLA-DR (MHC Class II) expression in PMNs in cellular interactions in culture from blood samples with positive Chagas serology with LPS stimulation.
2. Materials And Methods
Heparinized human blood samples were collected with ethical consent according to procedures approved by ethical comitee of National Hospital Clinicas R: 107/12. Samples donated by the Blood Bank, Institute of Hematology and Hemotherapy of the National University of Cordoba in anonymity, with negative serology: Hudleson (Wiener), VDRL (Wiener), Chagas HAI (Wiener) Chagas EIE (Biomerieux), HBs EIE (Biomerieux), HBc (Biomerieux), HCV EIE (Murex), HIV Ac EIE (Biomerieux), HIV Ag EIE (Biomerieux), HTLV EIE (Murex). Individuals considered “healthy” (H) n = 10; and only those with positive serology for Chagas (CH) n = 6 HAI (+) and EIE (+).
Blood samples were allowed to sediment for 2 hours. Plasma supernatants containing all types of leukocytes were separated and were cultured at 37 °C in TC199 medium (Sigma, St-Louis, MO) supplemented with L-glutamine (Sigma, St. Louis, MO), added with filtered serum from the same donor. A 24-well cell culture plate was prepared by putting a sterile 13 mm round glass cover slip into each well.
Cultured cells were stimulated with LPS (Lipopolysaccharides from Escherichia coli, Sigma-Aldrich) 25 ng/ml at 37 oC in a CO2 incubator. Samples: 30 minutes.
Glass cover slips with attached cells were carefully removed from culture plate and IF techniques were performed.After stimulation with LPS, culture cells washed briefly in PBS (phosphate buffered saline), fixation was performed with 4% paraformaldehyde for 10 minutes and washed in three changes in PBS. It was incubated with 5% blocking serum albumin in PBS to prevent non-specific staining for 20 minutes. It was washed with PBS. It was incubated with antibodies (Ab) Santa Cruz Biotechnology anti-HLA DR (FITC; Santa Cruz Biotechnology) at 4 °C overnight. It was washed with PBS and nuclear staining with DAPI (4,6’-diamidino-2- phenylindole) (Sigma, St Louis, MO). It was mounted with mounting medium 90% glycerol in PBS. Observation of preparations was done in videomicroscope Axioscop 20, MC80, trinocular, Carl Zeiss. Paired blood samples provided controls.
Cell pellets were prepared with samples with aliquots of crops grown. They were fixed in 1% glutaraldehyde in 0.1 M cacodylate buffer for one hour and post fixed in 1% OsO4 in the same buffer for one hour. Then the materials were dehydrated in increasing graduation acetone and embedded in epoxy resin (Araldite) at 60 oC for 24-48 hours. Later, ultrathin sections 60 were made to 80 nm thick (color silver / gold interference) that were collected on copper grids of 250 bars per inch, contrasted with uranyl acetate and lead citrate, and studied with electron microscope Zeiss LEO-906E transmission.
3. Statistical Analysis
The data was presented as the mean value ± SD, and represented at least three independent experiments from independent donors. Student t test for independent samples and Student t test for paired samples was applied for data analysis **p < 0.001. The difference between means was analyzed using Infostat software.
4. Results And Discussion
In samples without LPS stimulation HLA-DR was observed in few cells in both groups: healthy and with serology positive for Chagas disease.(Figure 1). Positive HLA-DR cells probably are B lymphocytes and monocytes because MHC Class II molecules are constitutively expressed. Moreover, in samples with serology positive for Chagas disease more cells positive HLA-DR could be T activated cells.
Cultured cells were stimulated with LPS and we marked HLA-DR. These molecules are detected on the cell surface at 30 minutes. In the control unstimulated paired blood samples at 30 min HLA-DR was found stained in some cells. LPS stimulation induced expression of HLA-DR molecules in PMNs in both groups (Figure 1) and samples with positive Chagas serology showed a higher number of HLA-DR + lymphocytes and HLA-DR + PMNs (p < 0.0001, t-test for paired samples and for independent samples). (Figure 1, Q-S). It has been described leukocytosis in some samples with patients with Chagas acute disease. We work with samples from asymptomatic people with positive serology for Chagas and this correspond to chronic stage, without leukocytosis. The presence of cytokines and inflammatory mediators in donor serum with Chagas-positive serology could induce the expression of HLA-DR molecules in addition to LPS stimulation. This finding could involve MHC Class II antigen presentation pathway to CD4+ T lymphocytes.
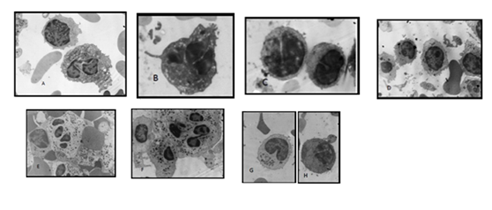
Cell-cell interactions between PMNs and lymphocytes were observed predominantly in samples with positive serology for Chagas disease. In this way the expression of HLA-DR in PMNs could involve MHC Class II antigen presentation pathway to CD4+ T lymphocytes. In the same way, lymphocytes showed abundant cytoplasm, and polarization of mitocondria, revealing signs of cellular activation. (Figure 2).
We observed altered mitochondrial morphology in lymphocytes of samples from both groups, healthy and with serology positive for Chagas and LPS stimulated, with electro lucid images (Figure 2). These lymphocytes do not show features of apoptotic cells and further study is necessary to research the possibility of pyroptosis [18]. Pyroptosis require activation of caspase-1/11, and caspase 11can be actívated by LPS. Specific markers for pyroptosis like lactate dehydrogenase release detected by enzyme assay, are useful.
Mitochondria are currently considered to have innate and adaptive immunity regulatory functions [20], support cell function and are determinants of the phenotypes that immune cells adopt in their responses [20]. It is known that endotoxemia produced by the effects of endotoxins such as LPS in the bloodstream leads to inflammation in multiple organs. T lymphocytes respond innately to LPS with signaling via the TLR4 receptor. LPS does not affect the proliferation or cytokine secretion of T lymphocytes but increases adherence and inhibits their migration [19].
5. Conclusion
The ultrastructural aspects reveal signs of cellular activation in chagasic samples and interactions between PMNs and lymphocytes. Expression of HLA-DR in PMNs could involve MHC ClassII antigen presentation pathway to CD4+ T lymphocytes.
References
- Abbas AK, Lichtman AH, Pillai S. (2017). Cellular and Molecular Immunology. 9th Edition Elsevier, Philadelphia, PA. P608.
- Murphy K, Weaver C. (2017). Janeway Immunobiology, 9th edition, Garland Science, Taylor and Francis Group. NY and London.
- Fainboim L, Geffner J. (2011). Introducción a la Inmunología Humana. 6ta. Edición. Editorial Médica Panamericana. Buenos Aires.
- Sandilands GP, Ahmed Z, Perry N, Davison M, Lupton A, et al. (2005) Cross-linking of neutrophil CD11b results in rapid cell surface expression of molecules required for antigen presentation and T-cell activation. Immunology 114: 354-68.
- Sandilands GP, McCrae J, Hill K, Perry M, Baxter D (2006) Major histocompatibility complex class II (DR) antigen and costimulatory molecules on in vitro and in vivo activated human polymorphonuclear neutrophils. Immunology 119: 562-71.
- Bern C, Martin DL, Gilman RH (2011) Acute and congenital Chagas disease. Adv Parasitol 75: 19-47.
- Ferreira LR, Frade AF, Baron MA, Navarro IC, Kalil J, et al. (2014) Interferon-γ and other inflammatory mediators in cardiomyocyte signaling during Chagas disease cardiomyopathy. World J Cardiol 6: 782-90.
- Tosello-Boari J, Vesely MCA, Bermejo DA, Ramello MC, Montes CL, et al. (2012) IL-17RA Signaling Reduces inflammation and mortality during Trypanosoma cruzi infection by recruiting suppressive IL- 10-producing neutrophils. PLoS Pathog 8(4): e1002658.
- Giraldo NA, Bolaños NI, Cuellar A, Roa N, Cucunuba Z, et al. (2013) T Lymphocytes from Chagasic Patients Are Activated but Lack Proliferative Capacity and Down-Regulate CD28 and CD3f. PLoS Negl Trop Dis 7(1): e2038. doi:10.1371/journal.pntd.0002038
- Cabral HR, Novak IT, Glocker M, Castro-Viera GA (2005) Neomicrovasculatura: factor activo en la inmunopatogenia de la cardiopatía crónica chagásica [Neomicrovasculatura: active factor in the immunopathology of chronic Chagas disease]. Rev Argent Cardiol 73: 201-7.
- Bonney KM, Engman DM (2008) Chagas heart disease pathogenesis: one mechanism or many? Curr Mol Med 8: 510-8.
- Rodriguez FM, Orquera AD, Maturano MR, Infante NS, Carabajal- Miotti C, et al. (2016) Human neutrophils in patients with positive serology for Chagas disease. J Immunol Infect Dis 3(1): 101.
- Cabral HRA (1987) Neutrophils with ring-shaped nuclei in human Chagas’ disease. Br J Haematol 67: 118-9.
- Cabral HR, Robert GB (1989) Ring-shaped nuclei in human neutrophilic leukocytes of healthy individuals: evidence of their occurrence and characteristics. Am J Hematol 30: 259-60.
- Rodriguez FM, Orquera AD, Maturano MR, Infante NS, Carabajal- Miotti C, et al. (2016) Human neutrophils in patients with positive serology for Chagas disease. J Immunol Infect Dis 3(1): 101.
- Rodríguez FM, Vargas A, Carabajal Miotti C, González Silva N, Ruiz de Frattari, S and Novak ITC (2017). Extracellular Traps and costimulatory molecules in leukocytes of patients with positive serology for Chagas disease. MOJ Immunol 2017. 5(4):00163. DOI 10.15406/moji.2017.05.00163.
- Rodríguez FM and Novak ITC (2016) Costimulatory molecules CD80 and CD86 colocalized in Neutrophil Extracellular Traps (NETs).. J Immunol Infect Dis 2016. 3(1): 103.
- Aachoui Y, Sagulenko V, Miao EA, Stacey KJ (2013). Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol. 2013 June ; 16(3): 319–326. doi:10.1016/j.mib.2013.04.004
- Zanin-Zhorov A, Tal-Lapidot G, Cahalon L, Cohen-Sfady M, Pevsner-Fischer M, Lider O, Cohen IR. (2007) Cutting Edge: T Cells Respond to Lipopolysaccharide Innately via TLR4 Signaling. 1 J Immunol 179:41-44; doi: 10.4049/jimmunol.179.1.41
- Weinberg SE, Sena LA, Chandel NS (2015) Mitochondria in the regulation of innate and adaptive immunity. Immunity. 42(3): 406–417. doi:10.1016/j.immuni.2015.02.002