Information
Journal Policies
Immunogenicity of a Modified Microcrystalline Tyrosine (MCT®)-Adsorbed Ragweed Immunotherapeutic Product with Monophosphoryl Lipid A in a Murine Model
J.W.Hutchings1,M.D.Heath2,M.A.Skinner3
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Specific immunotherapy has proven to be effective treatment for pollinosis which underlies clinical conditions such as allergic rhinitis, rhinoconjunctivitis and allergic asthma. The tolerability and activity of immunotherapeutic preparations can be improved using adjuvant systems such as the novel combination of L-tyrosine adsorbates (Microcrystalline Tyrosine; MCT®) with the addition of a Toll like receptor 4 (TLR-4) agonist monophosphoryl lipid A (MPL®). This study investigated the immunogenicity of the adjuvant formulation when combined with a candidate modified ragweed immunotherapeutic product.
Mice were immunised with a Ragweed modified allergen L-Tyrosine (MCT®)-adsorbate (MATA) with MPL®, in addition, control groups were immunised with MCT® or MPL® in the absence of the allergoid. Ragweed specific murine IgG1 and IgG2a was determined.
The presence of low dose versus high (therapeutic) dose MPL in the Ragweed MATA therapeutic was found to enhance Ragweed specific IgG1 and IgG2a antibody serum titre responses in the murine model in a step wise fashion. These findings demonstrate that the combination of MCT® with MPL® in the candidate Ragweed immunotherapeutic product generates high and sustained IgG antibody titres.
Modified Microcrystalline,Tyrosine (MCT®)-Adsorbed Ragweed,Monophosphoryl Lipid,Immunology and Vaccines
1. Introduction
Ragweed pollinosis is well documented in both the United States and Europe with 26.2% of an American sample population shown to give positive skin prick test reactions to short Ragweed pollen [1]. The clinical manifestations of this sensitisation can be commonly shown as rhinitis, rhinoconjunctivitis and in severe cases allergic asthma. The benefits of specific immunotherapy for the treatment of rhinoconjunctivitis compared to symptomatic treatment has been demonstrated clinically and offers sustained effects and a decreased risk of developing the more severe allergic asthma [2,3]. Treatment of pollinosis with allergoids absorbed to MCT® has been shown to be safe and efficacious, both with and without the adjuvant MPL® [4]. The immunogenicity study described in this paper further characterises the efficacy of these allergoids absorbed to MCT® with MPL®, through the measurement of specific immunoglobulin subtypes.
In Ragweed MATA MPL, Ragweed is a pollen (Ambrosia artemisiifolia) allergen extract is chemically modified with glutaraldehyde. Glutaraldehyde reacts with primary amino groups on specific amino acid residues and causes covalent cross linking and agglutination of the heterogeneous mixture of proteins in the extract. This results in the formation of a high molecular weight complex termed an allergoid [5]. During modification of allergen to allergoid there is a reduction in conformational IgE binding and B cell epitopes whilst there is retention of sequential IgG epitopes. This loss of IgE epitopes is due to the well documented conformational nature of IgE epitopes in allergens, where it has been demonstrated that IgE reactivity is highly dependent on allergen structure [6-8]. This modification of the allergen structure disrupts the conformational IgE epitopes thus reducing IgE reactivity of the allergoid in comparison to the native allergen [5]. In contrast IgG epitopes consist in a proportion of contiguous amino acid residues and are therefore less influenced by a change in protein conformation [9-11].
During manufacture, the modified allergen extract (allergoid) is adsorbed to L-tyrosine, a naturally occurring amino acid, in its microcrystalline form (MCT®) through proprietary manufacture processes. The relative insolubility under physiological conditions of L-tyrosine causes controlled release of the allergoid from MCT allergoid complex in vivo [12]. This controlled, slow-release, is thought to reduce the incidence of local IgE mediated allergic responses, whilst prolonging the antigenic response of the therapy [13-16]. MCT® also offers intrinsic adjuvant (Th1) properties and has been previously shown to function in synergy with MPL® in allergy immunotherapy and off physicochemical compatibility (adsorption) [15,16]. In the Ragweed MATA MPL formulation in addition to the MCT® allergoid complex the novel adjuvant MPL® is added. MPL® is derived from lipid A of Salmonella Minnesota R 595. Lipid A is an activator of the innate immune system through the activation of cognate receptors [18]. MPL® is derived from this lipid by the removal of a phosphate and fatty acid group which causes retention of a potent immunological effect whilst significantly reducing toxicity. The adjuvant activity of MPL® promotes primarily a T helper type 1 response through stimulation of the innate immune response by binding to the TLR4 complex [19]. This T helper type 1 response is indicative of immunological switching away from the IgE mediated allergic response, seen as the objective of specific immunotherapy [20].
Other adjuvants are used in specific allergen immunotherapy formulations. The most common of these is the use of aluminium salts, notably aluminium hydroxide. Aluminium hydroxide is a mineral adjuvant with substantial clinical experience gained through over 80 years of use as originally documented by Glenny., et al [21]. As a result, many subsequent prophylactic vaccine preparations were formulated containing aluminium salts on this basis [22,23]. However, there is increasing data suggesting that while a T helper type 1 response is generated by the aluminium hydroxide adjuvanted formulation, there is also induction of predominant T helper 2 responses [24]. Whilst long-standing evidence is available supporting the efficacy of aluminium salts in specific immunotherapy, this mechanism would indicate that aluminium salts may not be appropriate for use in allergy treatment where effective treatment is marked by a modulation of the immune system from a specific T helper 2 to a T helper 1 response. Moreover, due to aluminium’s non-biodegradable nature, research to identify suitable biodegradable alternative depot adjuvants is encouraged [25].
In this article, we demonstrate the in vivo immunological effect of administration of a modified Ragweed MATA pollen extract in the presence of a low dose and (therapeutic) high dose MPL® in a murine model.
2. Materials And Methods
39 female BALB/c mice, approximately 9 weeks old at day 0 of the study were acclimatised for a period of 7 days under observation. For the entirety of the study mice were caged with autoclaved sawdust bedding material under a 12 hour light, 12 hour dark cycle. On day 0, mice were randomly allocated into 4 groups with 10 mice per a group. The subjects were then immunised with 0.2ml the corresponding study material (refer to table 1) subcutaneously into the inguinal area of the mouse. 5 mice from each group were scarified and bled on study day 19. On study day 20 the remaining mice in each group received a repeat dose of 0.2ml of the corresponding study material as previous. All remaining mice were scarified at day 34. Blood samples collected at both testing points were refrigerated and allowed to clot overnight before centrifugation. Sera for each bleed was separated and stored at - 70°C to -90°C and subsequently dispatched to the testing laboratory packed with dry ice. The study was conducted at Charles River Laboratories Biolabs Europe (Co. Mayo, Ireland). All procedures were prepared by the Charles River Laboratories Biolabs Europe study director. Protocols were approved by Charles River Laboratories Biolabs Europe head of QA and the study sponsor and conform to European regulations for animal experimentation [26].
All formulations were manufactured by Allergy Therapeutics (UK) Ltd (United Kingdom) and are detailed below in table1:
The allergoid contained in the study material administered to groups A-B is manufactured from short Ragweed pollen (A Artemisiifolia) aqueous extraction at the same weight/volume ratio of 5%. The MATA MPL therapeutics are given as a 0.5ml dose when administered clinically in humans, therefore 25µg and 50µg dose refer to 50µg/mL and 100µg/mL MPL® content.
Specific IgG1, and IgG2a titre reactivity of samples was determined using a solid phase, direct uptake ELISA system. IgG1 and IgG2a determinations were performed for each serum sample at each time point. A Ragweed native antigen produced from an aqueous extract (Allergy Therapeutics (UK) Ltd) was used to coat the wells of a EIA Linbro microtitre plate (MP Biomedicals, UK). After overnight incubation at 2-8°C of the coating antigen and subsequent washing of the microtitre plate, sera samples were added and the plate incubated. Unbound sera was washed from the microtitre plate and assay specificity determined by the addition of anti-murine IgG1 or IgG2a secondary antibody conjugated to horse radish peroxidase enzyme (Research Diagnostics Inc., USA). After incubation and subsequent washing relative antibody titre was visualised through addition of TMB substrate system (BD Bioscience, USA). The resulting colour formation was stopped with the addition of 0.4M sulphuric acid and the plate read at 450nm using a microtitre plate reader. Ragweed specific IgG1 and IgG2a responses were determined per sample and a mean average plotted per study group and time point.
3. Results
The immunological responses for the two IgG subtypes was measured using ELISA based methods described above and data is presented for comparison of response against animal groups, which received different therapeutic formulations as described in table 1.
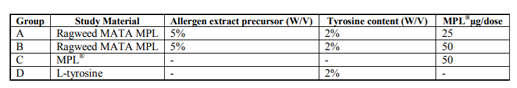
As seen in fig 1, groups A and B treated with the MPL® containing Ragweed MATA product presented high and sustained Ragweed specific IgG1 titres compared with control groups. Group B which was treated with the product containing 50µg/dose of MPL® exhibited the highest IgG1 titre. Group A, which was treated with the product containing 25µg/dose MPL®, exhibited a step-wise lower response whereby, at a dilution of 1/200, the 19 day bleeds exhibited a mean response that was 40% compared with group B at the same dilution and time point. For group A the 34 day bleeds, at a dilution of 1/6400, produced a mean response 60% of that given for group B at the same dilution and time point. For groups C and D treated with a product containing no allergoid, the mean Ragweed specific IgG1 titre responses were negligible.
Study material: (A) Ragweed MATA MPL® at 25µg/dose MPL, (B) Ragweed MATA MPL® at 50µg/dose MPL®, (C) 50µg/dose MPL®, (D) MCT® (2% w/v). Error bars denote standard error determined from all samples tested.
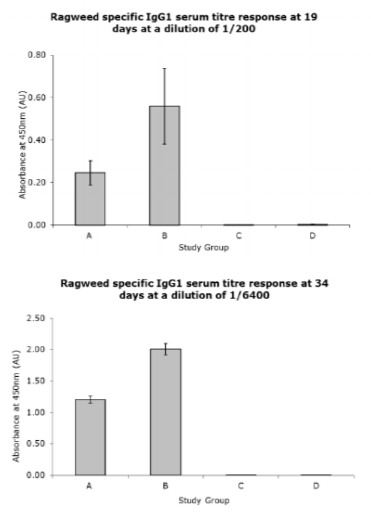
The increase in Ragweed specific IgG reactivity is further demonstrated in the Ragweed specific IgG2a titre measurements as detailed in fig 2. The groups treated with the material containing MPL produced high and sustained antibody titres. The Ragweed specific IgG2 titre response between the two groups treated with MPL® containing products was similar to that seen in the Ragweed specific IgG1 titre responses. Group B, that contains the therapeutic dose of MPL® at 50µg/dose, generated the largest Ragweed specific IgG2a titre response. Group A, treated with the product containing 25µg/dose MPL® produced a mean Ragweed specific IgG2a titre response 92% of that of group B and at 34 day bleed produced a mean response 65% of that presented in group B. As seen in the Ragweed specific IgG1 titre measurements, the non allergoid containing groups C and D elicited a small mean Ragweed specific IgG2 titre response in comparison to the active groups A-B.
Study material: (A) Ragweed MATA MPL at 25µg/dose MPL®, (B) Ragweed MATA MPL at 50µg/dose MPL®, (C) 50µg/dose MPL®, (D) MCT® (2% w/v). Error bars denote standard error determined from all samples tested.
4. Discussion
The immunological data reported in this study presents high and sustained Ragweed specific IgG1/IgG2a titres between the study group’s dependant on presence of allergoid and concentration of MPL®. The lower MPL® dose Ragweed MATA MPL (group A) produced a lower Ragweed specific IgG1/IgG2a titre compared with the top therapeutic strength MPL® dose group B. It should be noted that that the top dose 50 µg/mL of MPL® is what is used in commercial immunotherapy formulations. The evidence of MPL® presence, in immunotherapeutic formulations, increasing the IgG response to allergen is coherent with the findings of the clinical trial described in Patel., et al and in other immunotherapies [4,27-28]. The clinical efficacy of Ragweed MATA MPL was demonstrated during administration of the therapeutic course. This was indicated by improved total symptom score both pre- and post-controlled Ragweed pollen exposure using an environmental exposure chamber, improved rhinoconjunctivitis quality of life questionnaire results and through immunogenicity analysis. The trial comprised of 3 study groups with Ragweed MATA MPL, Ragweed MATA and MCT® as the study materials. The immunogenicity results mirrored the findings reported here by displaying average increases in Ragweed specific IgG, IgG1 and IgG4 serum titres several magnitudes higher than in the placebo group, treated with MCT in the absence of allergoid and MPL. The increased Ragweed specific IgG, IgG1 and IgG4 serum titre in humans as Patel., et al (4) and the increase in Ragweed specific IgG1 and IgG2a serum titre in the murine model as reported here, is indicative the enhanced T helper type 1 response that is documented when MPL® is used in candidate immunotherapies [29-32].
The results reported here show that the addition of MPL® to the MCT® allergoid complex induces a substantial increase in IgG1 and IgG2a serum titre indicating that the presence of MPL can increase the immunogenicity of an immunotherapeutic formulation. There is a clear stepwise increase in IgG1 and IgG2a titres seen when the subjects are administered with MCT®-absorbed Ragweed allergoid with a low dose of the TLR-4 agonist MPL® (25 µg/mL) and MCT®-absorbed Ragweed allergoid with a therapeutic dose of the MPL® (50 µg/mL), respectively. These findings demonstrate that the inclusion of the Th1 stimulating proprietary adjuvant, MPL®, improves the immunological profile of such candidate therapies.
Acknowledgement
We thank Alva Trimble, Ann Murray, and Geraldine Reilly at Charles River Laboratories Biolabs Europe, Co. Mayo, Ireland for in vivo animal experimentation studies. We thank Dr Lyuba Mikholovska, School of Pharmacy and Biomolecular Sciences, University of Brighton, UK for support in development and interpretation of immunoassays.
References
- Arbes SJ, Gergen PJ, Elliott L et al. Prevalence’s of positive skin test responses to 10 common allergens in the US population: Results from the Third National Health and Nutrition Examination Survey. J of Allergy and Clin Immunol 2005; 116(2):377-83.
- Jacobsen L, Niggemann B, Dreborg S et al. (The PAT investigator group). Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy 2007; 62(8):943-8.
- Möller C, Dreborg S, Ferdousi HA et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study). J of Allergy and Clin Immunol 2002; 109(2):251–6.
- Patel P, Holdich T, Fischer von Weikersthal-Drachenberg KJ et al. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J of Allergy and Clin Immunol 2013; 05.032. [Epub ahead of print]
- Starchenka S, Bell AJ, Mwange J, Skinner MA, and Heath MD. Molecular fingerprinting of complex grass allergoids: size assessments reveal new insights in epitope repertoires and functional capacities. World Allergy Organ J. 2017; 10(1): 17.
- Mirza O, Henriksen A, Ipsen H et al. Dominant Epitopes and Allergic Cross-Reactivity: Complex Formation Between a Fab Fragment of a Monoclonal Murine IgG Antibody and the Major Allergen from Birch Pollen Bet v 1. J Immunol 2000; 165(1):331-338.
- Gieras A, Cejka P, Blatt K et al. Mapping of Conformational IgE Epitopes with Peptide-Specific Monoclonal Antibodies Reveals Simultaneous Binding of Different IgE Antibodies to a Surface Patch on the Major Birch Pollen Allergen, Bet v 1. J Immunol 2011; 186(9):5333-5344.
- Schein CH, Ivanciuc O, Midoro-Horiuti T et al. An Allergen Portrait Gallery: Representative Structures and an Overview of IgE Binding Surfaces. Bioinform Biol Insights 2010; 11(4):113-25.
- Sela M, Schechter B, Borek F et al. Antibodies to Sequential and Conformational Determinants. Cold Spring Harbour Symp Quan Biol 1967; 32: 537-545.
- Arnon R, Van Regenmortel MH. Structural basis of antigenic specificity and design of new vaccines. FASEB J 1992; 6(14):3265-74.
- Baldrick P, Richardson D, Woroniecki SR et al. Pollinex® Quattro Ragweed: safety evaluation of a new allergy vaccine adjuvanted with monophosphoryl lipid A (MPL®) for the treatment of ragweed pollen allergy. J Appl Toxicol 2007; 27(4):399–409.
- Baldrick P, Richardson D, Wheeler AW. Review of L-tyrosine confirming its safe human use as an adjuvant. J Appl Toxicol. 2002; 22(5):333-44.
- KT, Stefura WP. Antigen-specific inhibition of ongoing murine IgE immune responses. II. Inhibition of IgE responses induced by treatment with glutaraldehyde-modified allergens is paralleled by reciprocal increases in IgG2a synthesis. J Immunol 1991; 147(8):2455-2460.
- Yang X, Gieni RS, Mosmann TR et al. Chemically modified antigen preferentially elicits induction of Th1-like cytokine synthesis patterns in vivo. J Exp Med 1993; 178(1):349-53.
- Wheeler AW, Marshall JS, Ulrich JT. A Th1-Inducing Adjuvant, MPL Enhances antibody profiles in experimental animals suggesting it has the potential to improve the efficacy of allergy vaccines. Int Arch Allergy Immunol 2001; 126:135-9.
- Bell AJ, Heath MD, Hewings SJ, Skinner MA. The adsorption of allergoids and 3-O-desacyl-4'-monophosphoryl lipid A (MPL®) to microcrystalline tyrosine (MCT) in formulations for use in allergy immunotherapy. J Inorg Biochem. 2015. 152:147-53.
- Wheeler AW, Moran DM, Robins BE et al. L-Tyrosine as an Immunological Adjuvant. Int Arch Allergy Immunol 1982; 69:113–119.
- Ishii KJ, Akira S. Toll or toll-free adjuvant path toward the optimal vaccine development. J Clin Immunol 2007; 27(4):363-71.
- Evans JT, Cluff CW, Johnson DA et al. Enhancement of antigen-specific immunity via the TLR4 ligands MPL™ adjuvant and Ribi.529. Expert Rev Vaccines 2003; 2(2):219-29.
- Patel P, Salapatek AM. Pollinex Quattro: a novel and well-tolerated, ultra short-course allergy vaccine. Expert Rev Vaccines 2006; 5(5):617-29.
- Glenny AT, Pope CG, Waddington H et al. The antigenic value of toxoid precipitated by potassium alum. J Path Bact 1926; 29:38-39.
- Norman W, Baylor NW, Egan W, R et al. Aluminum salts in vaccines—US perspective. Vaccine 2002; 20 Suppl 3:S18-23.
- Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev 1998; 32(3):155-172.
- Brewer JM, Conacher M, Hunter CA et al. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol 1999; 163(12):6448-54.
- Jensen-Jarolim E. Aluminium in Allergies and Allergen immunotherapy. World Allergy Organ J. 2015; 8(1): 7.
- European Directive 86/609/EEC on the protection of Animals used for Experimental and other scientific purposes.
- Till SJ, Francis JN, Nouri-Aria K et al. Mechanisms of immunotherapy. J of Allergy and Clin Immunol 2004; 113(6):1025–1034.
- Akdis CA, Blaser K. Mechanisms of allergen-specific immunotherapy. Allergy 2000; 55(6):522–530.
- Wheeler AW, Marshall JS, Ulrich JT. A Th1-Inducing Adjuvant, MPL Enhances antibody profiles in experimental animals suggesting it has the potential to improve the efficacy of allergy vaccines. Int Arch Allergy Immunol 2001; 126:135-9.
- Drachenberg KJ, Wheeler AW, Stuebner P et al. A well-tolerated grass pollen-specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy 2001; 56(6):498-505.
- Mothes N, Heinzkill M, Drachenberg KJ et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin Exp Allergy 2003 Sep; 33(9):1198-208.
- Drachenberg KJ, Heinzkill M, Urban E et al. Efficacy and tolerability of short-term specific immunotherapy with pollen allergoids adjuvanted by monophosphoryl lipid A (MPL) for children and adolescents. Allergol Immunopathol (Madr) 2003; 31(5):270-7.