Information
Journal Policies
Progress in Immunotherapy for Alzheimer’s disease-How to Overcome Recently Found Obstacles
Yoh Matsumoto,1,Kuniko Kohyama2
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Twenty years have passed since Schenk et al. developed peptide vaccines for Alzheimer’s disease (AD). However, subsequent clinical trials with active and passive immunization have failed to obtain sufficient outcomes to halt or improve cognitive decline. Other non-immunological therapies have also been unsuccessful in achieving satisfactory results. In this review article, we analyze factors regulating the results of these outcomes and look for ways in overcoming them. We also introduce recently developed DNA vaccines that targets both Aβ and tau deposits.
Alzheimer’s disease (AD), amyloid β (Aβ), tau, immunotherapy, DNA vaccine,Immunology and Vaccines
1. Introduction
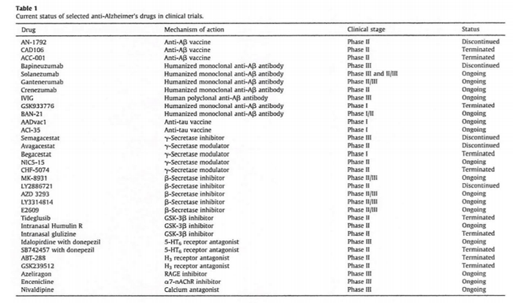
Alzheimer's disease (AD) is the most common cause of age-related dementia. The disease affects more than 12 million people worldwide and is characterized by progressive memory impairment and cognitive decline [1]. Senile plaques (Aβ deposition) and neurofibrillary tangles (hyperphosphorylated tau deposition) are two major hallmarks of AD pathology. Both Aβ and tau are neurotoxic and work independently or in combination to progress the disease. Based on the accumulated knowledge, investigators and clinicians have tried to develop immunotherapies for AD for the past two decades. As a result, active (peptide vaccines) and passive (monoclonal antibodies, mAbs) immunization against Aβ have been developed, and some have been used in clinical trials. However, at present, neither vaccines nor mAbs have showed satisfactory results. Although anti-Aβ vaccination was able to significantly reduce Aβ deposits in the brain of AD patients, no beneficial effects were observed with regards to cognitive decline. The status of various clinical trials is listed in Table 1 (cited from Ref [2] with permission).
In this article, we will briefly summarize the history of immunotherapy, analyze its obstable and then discuss the possibility for new immunotherapies.
2. Amyloid Cascade Hypothesis (Ach)
The theoretical background for immunotherapy is mainly based on the amyloid cascade theory (ACH). However, initial clinical trials using Aβ peptide vaccines (AN1792) raised doubt concerning this theory. Holmes et al. [3] reported that the autopsy of some AD patients who received the AN1792 vaccination had near complete depletion of Aβ deposits in the brain; however, these patients did not show any improvements in cognitive decline. These findings suggested that Aβ deposition is not a main player in AD pathogenesis. Despite this criticism, the accumulated evidence strongly suggests that Aβ deposition is an important prerequisite for the development of AD, which have been shown in clinical and experimental settings [4,5]. Ittner et al. demonstrated that enhanced redistribution of hyperphosphorylated tau from axons to the somatodendritic compartment during AD pathogenesis may increase tau-dependent sorting of Fyn to the dendrites, which results in the boosting of excitotoxic signaling and increase in the toxic effects of Aβ on neurons [6]. This group of proteins has been termed “Fyn-tau-amyloid toxic triad” [7]. Recent progress in ACH is well summarized in a review article [8].
3. Immunotherapy Targeting Aβ
In 1999, Schenk et al. demonstrated that monthly inoculation with a synthetic Aβ peptide vaccine could lead to high anti-Aβ antibody titers and dramatic reductions of Aβ deposition in PDAPP transgenic mice 9. Subsequent studies demonstrated that clearance of Aβ deposits following immunization protected APP - transgenic (Tg) mice from developing memory deficits[10,11]. When developing antibody - mediated immunotherapy either using vaccines or mAbs, it is important to determine whether the antibody targets intra- or extracellular components of neuronal cells; however, only few antibodies are known to penetrate the cell membrane and function intracellularly. Recently, it was demonstrated that Aβ [12,13] and tau [14]play a prion-like role in the formation of AD pathology. Certain types of Aβ and tau, such as pyroglutamate Aβ (AβpE3-42) and hyperphosphorylated tau, induce the misfolding and aggregation of normal proteins. These results suggest that antibodies that function against extracellular targets can be developed against these molecules. Recent findings regarding prions are summarized by Collinge [15].
4. Passive Immunization
Immunotherapies have become focused on passive immunization using mAbs after the clinical trials with the anti-Aβ vaccine, AN1792, were stopped due to the development of meningoencephalitis in some treated patients. Recent clinical trials for active and passive immunizations are listed in Table 1 (cited from Ref.2 with permission). Although bapineuzumab, solanezumab and other mAbs were employed in clinical trials to treat mild to moderate AD, none of them showed satisfactory results [16-18]. Based on these results, mAbs were subsequently used in prevention trials such as DIAN, A4 [19] and API [20] (Table 1). However, currently, there are no reports suggesting the marked preventive effects of mAb treatment. Furthermore, Eli Lilly abandoned solanezumab as treatment for mild dementia [21]. Very recently, it was reported that aducanumab, an mAb which selectively targets the aggregated Aβ, reduced Aβ plaques and slowed cognitive decline in phase I clinical trials [22]. However, it remains to be undetermined whether this treatment is effective in improving cognitive decline [23]. Furthermore, evidence that aducanumab is superior to previous mAbs, such as bapineuzumab and solanezumab, is not available at the present time.
These results raise at least two possibilities. First, targeting only Aβ may not be sufficient enough to halt or improve cognitive decline; this will be discussed in detail below. The second relates to the antigen specificity of mAbs. Furthermore, it is unlikely that the stage of AD is important when starting treatment since the prevention study did not show favorable outcomes.
There is little known concerning the antigen specificity of anti-Aβ mAbs, which were used in clinical trials. The linear sequence recognized by each mAb is known, but it is still unclear whether or not mAbs recognize conformational epitope(s) of various Aβ species. Bapineuzumab, solanezumab, gantenerumab and crenezumab have been reported to bind Aβ monomers, oligomers and fibrils; however, most mAbs were unable to achieve beneficial effects in clinical trials [24]. We have done an extensive survey of the literature and found only one paper regarding this topic. Watt et al. examined the binding ability of clinically used anti-Aβ mAbs and found that bapineuzumab, but not solanezumab and crenezumab, demonstrated target engagement of brain Aβ [25]. This result, however, was heavily criticized by Holzman’s group in terms of the techniques that were used [26]. Thus, further investigations are required to determine the degree of specificity of these mAbs. Unfortunately, bapineuzumab and solanezumab did not reach satisfactory endpoints in clinical trials, and their developments have been discontinued.
5. Active Immunization
Active immunotherapy seems to be more effective in reducing the A species compared with passive immunotherapy. Several autopsy reports demonstrated that some A vaccine (AN1792)-treated patients showed complete disappearance of A plaques [3,27,28]. Furthermore, AN1792 vaccination induced anti bodies against a wide variety of A species 29. As mentioned previously, the AN1792 treatment did not stop the progression of cognitive decline [3]. Boche et al. reasoned that the failure to halt cognitive decline by the AN1792 vaccine was due to its limitations in reducing aggregated tau in the neuronal process [28]. These results raise the possibility that Aβ depletion alone is not sufficient to halt cognitive decline.
CAD106 is a peptide vaccine comprising of Aβ1-6 coupling to the virus-like particle Oβ [30,31].
Phase 2/3 trials began in November 2015 and are set to continue until 2023 with a 5-year treatment period. This study aims to enroll 1,340 homozygous ApoE4 carriers between the ages of 60 and 75 who are cognitively normal (Alzforum, CAD106). Therefore, conclusions made at a future date will determine whether CAD106 is effective in improving or halting cognitive decline.
6. Anti-Tau Immunotherapy
Failure of the anti-Aβ immunotherapies in clinical trials, especially those using mAbs, prompted the development of anti-tau immunotherapy. Recent progress in this area is well summarized in review articles [32,33]. Both AADvac1 [34] and ACI-35 [35] are peptide vaccines and are currently in clinical trials. However, it is still early to evaluate the effects of these drugs. There is particular interest in determining whether anti-tau immunotherapy is effective for patients with early to moderate AD. As mentioned previously, anti-Aβ immunotherapy did not show satisfactory results at this stage of the disease. Furthermore, the tau sequence employed for immunotherapy seems to be important. In the experimental setting, Umeda et al. demonstrated that using several anti-tau mAbs, the anti-pSer413 antibody, but not the anti-pSer396 antibody, was effective in reducing tau deposits and improving memory [36]. Very recently, it was reported that tau immunotherapy inhibits not only tau but also Aβ pathology [37,38]. Since the effects of anti-Aβ immunotherapy on tau pathology is very limited [39,40], it is possible that anti-tau therapy provides more benefits than anti-Aβ therapy. Although beneficial effects such memory improvements in mice models are important, it does not guarantee the effectiveness of anti-tau vaccines and mAbs in clinical trials as experienced in the anti-Aβ immunotherapies.
7. Anti-Aβ (Ym3711 41) And Anti-Aβ/Tau (Ym7555 42) Dna Vaccines
To compensate the disadvantage of conventional immunetherapies, DNA vaccination has been developed as a new therapy for AD [43,44]. At the injection site, the vaccines are taken up by muscle cells and the Aβ peptide-protein complex is produced for a certain period [45]. Translated Aβ or Aβ/tau complex stimulates immune responses in the host, and induced anti-Aβ and/or tau antibodies. Importantly, immune responses of the host can be easily manipulated to obtain a Th2 type reaction [43,46,47].
Aβ oligomers as well as other Aβ species and amyloidogenic peptides are neurotoxic and play a pivotal role in AD pathogenesis [48]. In particular, it is important to remove conformationally abnormal structures through treatment. We attempted to develop new DNA vaccines and found that an IgL-Aβx4-Fc-IL-4 vaccine, designated as YM3711, was found to induce significantly higher levels of antibodies not only against Aβ1-42 but also AD-related molecules including AβpE3-42, Aβ oligomers and Aβ fibrils. Importantly, YM3711 significantly reduced these Aβ species in the brains of mice [48]. Thus, YM3711 is a powerful DNA vaccine targeting a wide range of AD-related molecules and is worth examining in clinical trials. Furthermore, we are attempting to develop more effective vaccines in a subsequent project.
In order to create more effective immune therapies for AD, we have developed new DNA vaccines that target tau alone or both Aβ and tau depositions[49]. Of these vaccines, YM7555 has four tandem - repeats of human Aβ1 - 42 and human tau 379 - 408 sequences that are connected to both ends of the Fc portion of immunoglobulin.
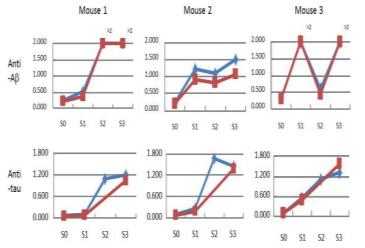
3x Tg and wild-type mice were injected biweekly with YM7555 at a dose of 100 μg/ injection in mice and 1 mg/injection in rabbits. Titers of anti-Aβ1-42 and anti-tau antibodies were determined by ELISA using plasma taken at the indicated time points.
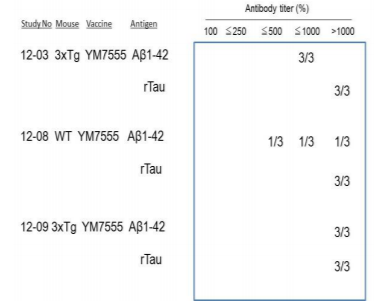
3x Tg mice were injected with YM7555 intramuscularly, and the kinetics of anti-Aβ (Figure 1A-C) and anti-tau (Figure 1D-F) antibody titers were determined by ELISA. The results of individual mice (n = 3) are shown. Both anti–Aβ and anti-tau antibody titers started to increase 4 or 8 weeks after the first vaccination and peaked at 8 or 12 weeks in all vaccinated mice. At the end of the study, anti–Aβ and anti-tau antibodies showed approximately a 10-fold increase. The results of the three studies are summarized in Figure 2. YM7555 immunization induced moderate (500-1000%) to high (>1000%) titers of anti-Aβ antibodies. Interestingly, the same YM7555 vaccination induced high titers of anti-tau antibodies in 3 x Tg mice. From these results, it is clear that the administration of the Aβ/tau vaccine (YM7555) induces a significant increase in antibodies against Aβ and tau (Figure 2) [49].
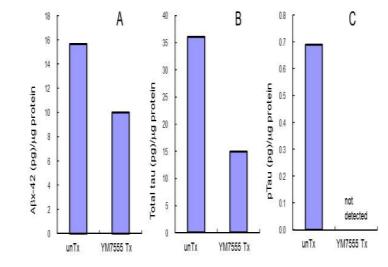
To assess the Aβ and tau reduction efficiency of Aβ/tau vaccines, 3x Tg mice were administered with YM7555 49. After repeated vaccinations, the levels of Aβ and tau in the cerebral cortex were quantified by sandwich ELISA. As shown in Figure 3, the amount of Aβ (panel A), total tau (panel B) and phosphorylated tau (panel C) was lower than untreated Tg mice. Importantly, phosphorylated tau, which is neurotoxic, was no longer detected after YM7555 vaccination (panel C) [49].
8. Discussion And Conclusion
Failures in improving cognitive decline by peptide vaccine (AN1792) and several anti-Aβ mAbs in clinical trials raised the possibility that these therapies are ineffective for patients with mild to moderate AD due to the late start in treatment. This prompted the anti-Aβ prevention trial for clinically normal individuals with a genetic predisposition for AD or who carry severe risk factors. Unfortunately, the prevention trials also showed unfavorable results, which strongly suggested that targeting only Aβ is not sufficient to obtain beneficial effects. The point is that Aβ reduction by the treatment showed little beneficial effects on tau pathology, thereby anti-Aβ immunotherapy did not halt cognitive decline in AD patients. Very recently, the A4 (the Anti-Amyloid treatment in Asymptomatic Alzheimer’s study) researchers are trying to overcome this obstacle by raising the solanezumab dosage (Alzform, June 29; 2017). It seems to be too optimistic to anticipate that this modification would be effective in prevention of AD development. As mentioned earlier, some species of Aβ and tau are neurotoxic alone or in combination. Taken all the situations into consideration, it is the time to start immunotherapy targeting both Aβ and tau. Although these studies are currently very preliminary, more focus should be given to vaccine projects that target Aβ and tau.
References
- Citron M: Alzheimer's disease: treatments in discovery and development. Nat Neurosci 2002, 5 Suppl: 1055-7.
- Godyn J, Jonczyk J, Panek D, Malawska B: Therapeutic strategies for Alzheimer's disease in clinical trials. Pharmacol Rep 2016, 68:127-38.
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA: Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet 2008, 372:216-23.
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ: Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002, 416:535-9.
- Moore S, Evans LD, Andersson T, Portelius E, Smith J, Dias TB, Saurat N, McGlade A, Kirwan P, Blennow K, Hardy J, Zetterberg H, Livesey FJ: APP metabolism regulates tau proteostasis in human cerebral cortex neurons. Cell Rep 2015, 11:689-96.
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wolfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Gotz J: Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell 2010, 142:387-97.
- Haass C, Mandelkow E: Fyn-tau-amyloid: a toxic triad. Cell 2010, 142:356-8.
- Selkoe DJ, Hardy J: The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 2016, 8:595-608.
- Schenk D, Barbour R, Dunn W, G. G, Grajeda H, Guido T, Hu K, Huang J, Johnson Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P: Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999, 400:173-7.
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW: A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature 2000, 408:982-5.
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D: A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature 2000, 408:979-82.
- Nussbaum JM, Schilling S, Cynis H, Silva A, Swanson E, Wangsanut T, Tayler K, Wiltgen B, Hatami A, Ronicke R, Reymann K, Hutter-Paier B, Alexandru A, Jagla W, Graubner S, Glabe CG, Demuth HU, Bloom GS: Prion-like behaviour and tau-dependent cytotoxicity of pyroglutamylated amyloid-beta. Nature 2012, 485:651-5.
- Jaunmuktane Z, Mead S, Ellis M, Wadsworth JD, Nicoll AJ, Kenny J, Launchbury F, Linehan J, Richard-Loendt A, Walker AS, Rudge P, Collinge J, Brandner S: Evidence for human transmission of amyloid-beta pathology and cerebral amyloid angiopathy. Nature 2015, 525:247-50.
- de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT: Propagation of tau pathology in a model of early Alzheimer's disease. Neuron 2012, 73:685-97.
- Collinge J: Mammalian prions and their wider relevance in neurodegenerative diseases. Nature 2016, 539:217-26.
- Tayeb HO, Murray ED, Price BH, Tarazi FI: Bapineuzumab and solanezumab for Alzheimer's disease: is the 'amyloid cascade hypothesis' still alive? Expert Opin Biol Ther 2013, 13:1075-84.
- Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R: Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med 2014, 370:311-21.
- Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR, Bapineuzumab, Clinical Trial I: Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med 2014, 370:322-33.
- Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, Aisen P: The A4 study: stopping AD before symptoms begin? Sci Transl Med 2014, 6:228fs13.
- Ayutyanont N, Langbaum JB, Hendrix SB, Chen K, Fleisher AS, Friesenhahn M, Ward M, Aguirre C, Acosta-Baena N, Madrigal L, Munoz C, Tirado V, Moreno S, Tariot PN, Lopera F, Reiman EM: The Alzheimer's prevention initiative composite cognitive test score: sample size estimates for the evaluation of preclinical Alzheimer's disease treatments in presenilin 1 E280A mutation carriers. J Clin Psychiatry 2014, 75:652-60.
- Abbott A, Dolgin E: Failed Alzheimer's trial does not kill leading theory of disease. Nature 2016, 540:15-6.
- Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O'Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A: The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature 2016, 537:50-6.
- Reiman EM: Alzheimer's disease: Attack on amyloid-beta protein. Nature 2016, 537:36-7.
- Liu J, Yang B, Ke J, Li W, Suen WC: Antibody-Based Drugs and Approaches Against Amyloid-beta Species for Alzheimer's Disease Immunotherapy. Drugs Aging 2016, 33:685-97.
- Watt AD, Crespi GA, Down RA, Ascher DB, Gunn A, Perez KA, McLean CA, Villemagne VL, Parker MW, Barnham KJ, Miles LA: Do current therapeutic anti-Abeta antibodies for Alzheimer's disease engage the target? Acta Neuropathol 2014, 127:803-10.
- Siemers ER, Friedrich S, Dean RA, Gonzales CR, Farlow MR, Paul SM, Demattos RB: Safety and changes in plasma and cerebrospinal fluid amyloid beta after a single administration of an amyloid beta monoclonal antibody in subjects with Alzheimer disease. Clin Neuropharmacol 2010, 33:67-73.
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO: Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med 2003, 9:448-52.
- Boche D, Donald J, Love S, Harris S, Neal JW, Holmes C, Nicoll JA: Reduction of aggregated Tau in neuronal processes but not in the cell bodies after Abeta42 immunisation in Alzheimer's disease. Acta Neuropathol 2010, 120:13-20.
- Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinson D, Bayer A, Games D, Seubert P, Schenk D, Holmes C: Abeta species removal after abeta42 immunization. J Neuropathol Exp Neurol 2006, 65:1040-8.
- Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, Maguire RP, Blennow K, Lundmark J, Staufenbiel M, Orgogozo JM, Graf A: Safety, tolerability, and antibody response of active Abeta immunotherapy with CAD106 in patients with Alzheimer's disease: randomised, double-blind,placebo-controlled, first-in-human study. Lancet Neurol 2012, 11:597-604.
- Farlow MR, Andreasen N, Riviere ME, Vostiar I, Vitaliti A, Sovago J, Caputo A, Winblad B, Graf A: Long-term treatment with active Abeta immunotherapy with CAD106 in mild Alzheimer's disease. Alzheimers Res Ther 2015, 7:23.
- Rosenmann H: Immunotherapy for targeting tau pathology in Alzheimer's disease and tauopathies. Curr Alzheimer Res 2013, 10:217-28.
- Gruninger F: Invited review: Drug development for tauopathies. Neuropathol Appl Neurobiol 2015, 41:81-96.
- Kontsekova E, Zilka N, Kovacech B, Novak P, Novak M: First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer's disease model. Alzheimers Res Ther 2014, 6:44.
- Theunis C, Crespo-Biel N, Gafner V, Pihlgren M, Lopez-Deber MP, Reis P, Hickman DT, Adolfsson O, Chuard N, Ndao DM, Borghgraef P, Devijver H, Van Leuven F, Pfeifer A, Muhs A: Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS One 2013, 8:e72301.
- Umeda T, Eguchi H, Kunori Y, Matsumoto Y, Taniguchi T, Mori H, Tomiyama T: Passive immunotherapy of tauopathy targeting pSer413-tau: a pilot study in mice. Ann Clin Transl Neurol 2015, 2:241-55.
- Castillo-Carranza DL, Guerrero-Munoz MJ, Sengupta U, Hernandez C, Barrett AD, Dineley K, Kayed R: Tau immunotherapy modulates both pathological tau and upstream amyloid pathology in an Alzheimer's disease mouse model. J Neurosci 2015, 35:4857-68.
- Dai CL, Tung YC, Liu F, Gong CX, Iqbal K: Tau passive immunization inhibits not only tau but also Abeta pathology. Alzheimers Res Ther 2017, 9:1.
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM: Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 2004, 43:321-32.
- Boche D, Denham N, Holmes C, Nicoll JA: Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer's disease pathogenesis. Acta Neuropathol 2010, 120:369-84.
- Matsumoto Y: US9, 173,928 B2; EP201007 56204; JP5701747 and AU20102281 68. 2015.
- Matsumoto Y: This application has entered the national or regional phase in Japan, the U.S. and Europe. 2017.
- Tang DC, DeVit M, Johnston SA: Genetic immunization is a simple method for eliciting an immune response. Nature 1992, 356:152-4.
- Barry MA, Lai WC, Johnston SA: Protection against mycoplasma infection using expression-library immunization. Nature 1995, 377:632-5.
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL: Direct gene transfer into mouse muscle in vivo. Science 1990, 247:1465-8.
- Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A: Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 1993, 259:1745-9.
- Hoffman SL, Doolan DL, Sedegah M, Gramzinski R, Wang H, Gowda K, Hobart P, Margalith M, Norman J, Hedstrom RC: Nucleic acid malaria vaccines. Current status and potential. Ann N Y Acad Sci 1995, 772:88-94.
- Matsumoto Y, Niimi N, Kohyama K: Development of a new DNA vaccine for Alzheimer disease targeting a wide range of abeta species and amyloidogenic peptides. PLoS One 2013, 8:e75203.
- nternational Conference on Alzheimer's and Parkinson's diseases. Vienna, Austria, 2017. p.124.