Information
Journal Policies
Hybridomas Producing Monoclonal Antibodies against Tetanus Toxin Replaces the Polyclonal Horse Anti-Tetanic Serum
Seida A.A1*,Soliman R1
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Preparation of monoclonal antibodies against tetanus toxin and evaluation of its efficacy obtained by using spleen cells (B-Cells) which harvested from well immunized BALB/c mice were used, also myeloma cell lines P3NS/1 were used. Fusion between spleen cells and myeloma cell lines occurred through fusogenic material called PEG 1500, 40% concentration to produce hybridomas which had the characteristic of both parents the ability to grow indefinitely from myeloma and the ability to produce of specific antibody from the B-cells. In this study we succeed to produce five hybridomas producing monoclonal antibodies against tetanus toxin and also succeed to be cloned into 5 stable clones producing monoclonal antibodies against tetanus toxin. Evaluation the efficacy of monoclonal antibodies against tetanus toxin through using indirect ELISA and evaluation the biological activity of produced monoclonal antibodies against tetanus toxin by using tetanus toxin neutralizing test in mice the survival rate reach 100% of the tested mice.
Hybridomas,Producing Monoclonal,Antibodies,Polyclonal Horse,Anti-Tetanic Serum,Immunology and Vaccines
1. Introduction
Clostridium tetani is the cause of tetanus in animals and man; spores are distributed throughout the world in soil, faeces, dust, clothing and many other situations. The organisms have often been isolated from human and animal faeces and they occur more frequently in richer soils, which have been fertilized with human and animal faeces (Buxton and Fraser, 1977).
Tetanus can affect many species of domesticated animals but occurs most frequently in horses and lambs, and less commonly in adult sheep, goat, cattle, pigs, dogs and cats. The disease occurs more rarely in poultry, which are relatively resistant to action of tetanospasmin (Carter (a), 1973).
Two toxic substances are produced, a hemolysin (Tetanolysine) and the potent lethal toxin (Tetanospasmin or Neurotoxin). The former is responsible for areas of hemolysis around colonies on blood agar plates. Neurotoxin is highly toxic when injected parentrally; however, it is harmless if administered by mouth. Animals vary in their susceptibility to tetanus toxin, e.g., horse and man are the most susceptible and cats are most resistant. These toxins are elaborated at the site of infection and passes directly to motor nerves and then to cord via the lymph and blood. These toxins act at the inhibitory synapses, where it blocks the normal function of the inhibitory transmitter (Carter (b), 1973 and Ahnert and Bigalke, 1995).
Clostridium tetani antitoxins (Polyclonal antibodies) prepared in horses, or sometimes in other domestic animals such as sheep or cows, is used in form of native serum or more commonly refined globulins (Pope and Porter, 1963).
The development of hybridoma technology by G. Kohler and C. Milstein revolutionized immunology after 1975. In 1984, Kohler and Milstein were awarded the Nobel Prize. These investigators demonstrated that antibody-producing cells of virtually any desired specificity could be fused with a myeloma cell line, the result being unlimited amounts of homogeneous (monoclonal) antibodies carrying that specificity(Stone, 2001).
Monoclonal antibodies have the obvious advantages of single specificity, and have been produced out of a need for homogenous antibodies as reproducible reagents. Polyclonal antibodies are a minor component in a complex mixture of serum proteins and are a hetero geneous mixture of molecules with a wide range of binding affinities (Hay and Westwood, 2002).
The methodology of monoclonal antibodies for preparation of tetanus antitoxin against tetanus toxin was generated by fusion of mouse NS-1 myeloma cells with spleen cells from BALB/C mice immunized with tetanus toxoid (Shang and Yeh, 1988).
Hybridoma technology is now being introduced to the study of bacterial toxins. Monoclonal antibodies (MABs) against specific epitopes on tetanus toxin could potentially replace the use of polyclonal antisera (Sheppard and Deborah, 1984).
2. Materials And Methods
The cell line used is P3NS1 synthesizes, but does not secrete, K light chain. Hybrids can secrete a mixed molecule of antibody heavy chains with myeloma light chain and myeloma kindly provided by the CLMAP-VACSERA.
Cells suspension derived from BALB/C mice, immunized with tetanus toxoid and tetanus toxin.
After intraperitoneal injection of BALB/C mice with incomplete Freund's adjuvant the mice peritoneal cavity was washed and peritoneal macrophages were collected and used as feeder cells to encourage the growth of hybrids.
BALB/C mice, kindly provided by the Experimental animal's farm in Helwan – VACSERA were kept under a good environmental and nutritional healthy condition. The BALB/C mice four week age were used for immunization with immunogen for production of immune spleen cells suspension after spleenoctomy. Adult mice are used for preparation of peritoneal macrophages used as feeder cells.
Complete Freund's adjuvant (CFA): used only for primary immunization (SIGMA). Incomplete Freund's adjuvant (IFA): used for booster immunization.
3. Estimation Of Total Protein Content Of Tetanus Toxoid And Tetanus Toxin By Using The Lowery Method To Determine Protein Concentration (Ausubel And Brent.2003)
The Lowry method depends on quantitating the color obtained from the reaction of Folin-Ciocalteu phenol reagent with the tyrosyl residues of an unknown protein and comparing this color value to the color values derived from a standard curve of a standard protein, usually BSA.
4. Preparation Of A Stable Emulsion Of Tetanus Toxin In Freund's Adjuvant
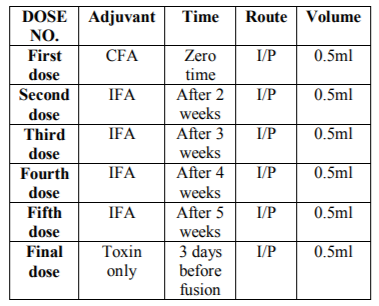
This protocol of immunization was performed with some modification according to (Davis et al.1983) and (Ausubel and Brent 2003). Five successive doses of the tetanus toxoid antigen kindly provided from CLMAP-VACSERA, 50 µg were introduced to the mice. First immunization of BALB/c mice used equal volume of antigen (Tetanus Toxoid) 50 µg and complete Freund's adjuvant (CFA) the injection volume was 0.5 ml/mouse Intraperitoneal injection (I/P). Second immunization after two weeks used an equal volume of antigen and incomplete Freund's adjuvant (IFA) were mixed will and used for the injection of immunized mice (0.5 ml/mouse I/P). Third, fourth and fifth immunization were similar to the second dose but with one week interval. Final boost 3 days before fusion the tetanus toxin was injected 0.5 ml I/P.
5. Preparation Of Myeloma Cells (Mohammad Ali 1994) And (Ausubel And Brent. 2003)
Cells were usually maintained in 250 ml culture flasks in 50 ml of complete culture medium with 20% FCS in 5% CO2, 37oC temperature and 98% relative humidity incubator, at density between of 107-108cells/ml but if increased than normal density occur over- crowding induce cell death. Prior to fusion it was essential that the P3NS1 myeloma cells were in the log phase of growth (mean over 95% of cells should be viable).
6. Elisa Screening Test (Ausubel And Brent.2003)
Predetermined maximal concentration of antigen was adsorbed to the microplate diluted in coating buffer and incubated (100 µl per well) overnight at 4oC also one row was coated with irrelevant protein as a negative control (1% BSA in coating buffer). Antigen solution was aspirated and blocked unbounded sites on wells by incubating 100 µl of blocking buffer per well for 30min-1 hr at 37oC. Unbounded protein was washed three times with washing buffer (PBs + TWEEN 20%). Test samples were added and incubated (100 µl per well) for one hour at 37oC. Unbounded antibodies were washed three times with washing buffer. Enzyme-labelled anti-mouse-Ig enzyme conjugate at the predetermined dilution was added and incubated (100 µl per well) for one hour at 37oC. Unbounded enzyme conjugates were washed three times with washing buffer. Enzyme substrate was added and incubated (100 µl per well) for one hour at 37oC until color develops (30min). About 9.50 µl of the stopping buffer was added and then assessed the plate either visually or by ELISA reader.
7. Polyethylene Glycol Aided Fusion (Bazin And Lemieux. 1988) And Mohammad Ali. 1994)
After 3 days of last immunization the spleen of mice was harvested and spleen cells were fused with the mouse myeloma cells at ratio 10:1. PEG 1500 0.9 ml 50% via 1ml pipette was added drop by drop over 1 min (Rotate tube in the hand). 1ml RPMI without serum was added drop by drop over 1 min with gentle agitation. Cell mixture was distributed in three 3 culture plates (180 well). Plates were leaved in 5% CO2, 37oC incubator overnight before adding HAT medium.
The 96-well plates were changed to HAT medium on the day following fusion (Day 2). Half the old Medium was removed and replaced with HAT medium (2ml HAT + 48 ml RPMI) (2x). This medium was changed by HAT medium (1ml HAT + 49 ml RPMI) (1x) repeated on day 4, 6,8,11 and every two to three days afterwards until the first screen. Within three to four days all the myeloma cells be dead, at fifth day hybrids were started to become visible. Specific antibody production (at day 11-14 day) was screened by ELISA, at least three days were allowed between screen and last medium change. After screening, positive antibody secreting wells were selected and expanded.
8. Expansion Of Culture Prior To Cloning
1ml HT medium were added to each 2ml well. Cells were suspended in positive 100µl well then transferred to 2ml well and mix. After two days feed with an additional 0.5ml of HT medium.
Plate 96 wells with average 5 cells per well (row A and B). Plate 96 wells with average 2 cells per well (row C and D). Plate 96 wells with average 1 cells per well (row E and F). Plate 96 wells with average 0.5 cells per well (row G and H). Positive wells were produced monoclonal antibodies were expanded, freezed and propagated in mice.
The biological activity of monoclonal antibodies against tetanus toxin was determined by using a tetanus toxin neutralization test: equal amounts (each of 0.5ml) of clones supernatant were perincubated with the toxin for one hour. Five mice were injected I/P by 0.2ml from the incubated mixture. Five mice were injected with 0.1ml I /P with toxin alone (Control group). The tested groups and the control one were observed up to 48 hours post inoculation for the survival of mice.
Mix equal volumes of 2% agarose and 6% PEG solutions. Keep in water bath at 55-60oC. Pour 5ml of agarose-PEG solution in plate. Punch holes in the agarose plate using the gel punch. Use 5µl of each isotype specific reagent in outer wells, and 5µl of test specimen in the center well. Place the agarose plate in a humidity chamber for at least 24 hours. The precipitin lines will appear in dark field light viewing box as dense,opaque white lines in the agarose layer.
9. Results
The total protein content of tetanus toxoid was 1.2 mg/ml.
The total protein content of tetanus toxins was 6.4 mg/ml.
The immunized mice were bled 7 days after the second immunization, serum was collected and the immune response to tetanus toxoid was measured using ELISA technique. Strong responder mice were selected and hyper immunized. Three mice were selected from the immunized group because of its good response, where the measured antibody titer was < 1/1000.
Cut off value of control negative serum samples was equal to 0.02
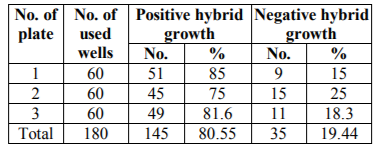
Seven to twelve days post fusion the three fusion plates (180 wells) were screened by microscopical examination of the bottom of the wells. Hybrid cell growth covering 10% to 50% of the surface area of the wells were selected. At 12 days post fusion the wells were screened for monoclonal antibodies production against tetanus toxin using ELISA.
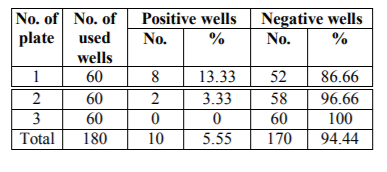
The first screening for anti-tetanus monoclonal antibody production was made 12 days post fusion on supernatants from the hybrid cell growth that covers 10% to 50% of the surface area of the wells. In these wells changing of the medium was stopped 4 days before screening. Results are shown in Tables NO. 5, 6 and7.
Summarized the results of screening for anti-tetanus monoclonal antibodies in the supernatants of wells showing hybrid growth using ELISA. Twelve days post fusion wells showing hybrid cell growth covering 10% to 50% of the surface area of wells was examined and positive hybridoma was detected and recovered.
Cloning of the recovered positive hydridomas using the limiting dilution technique was done to select a pure hybrid cells producing monoclonal antibodies against tetanus toxin. The main aim of cloning was to purify the positive hybridoma from non-producer cells.
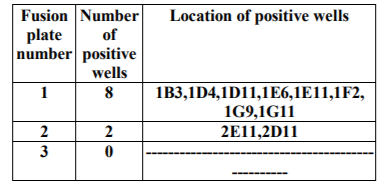
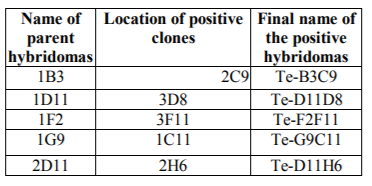
Cloning plates were examined microscopically and serologically using ELISA to select wells having single clones that produce anti-tetanus monoclonal antibodies. Five hybridomas, namely, 1B3, 1D11, 1F2, 1G9, 2D11 out of the ten were cloned successfully.
Indirect ELISA revealed that 5 hybridomas were successfully cloned and five stable clones were recovered, namely, 1B3, 1D11, 1F2, 1G9, 2D11.
10. Determination Of Neutralizing Efficacy Of The Monoclonal Antibodies Against Tetanus Toxin
The group of mice, which were inoculated with the mixture of the clones supernatant with the tetanus toxin showed 100% survival rate, as compared with the control group inoculated with toxin which manifest 0% survival rate.
11. Characterization Of The Monoclonal Antibodies Produced By The Five Hybridomas
Using agar gel procedure and mouse immunoglobulin isotyping kits, all of the five obtained clones have been identified and all were found to belong to IgM isotype.
12. Discussion
In the present work it was planned to produce monoclonal antibodies against the tetanus toxin and to characterize it as a preliminary step toward the preparation of monoclonal therapeutic or prophylactic product for tetanus toxin. Primary immunization of BALB/C mice was performed by intraperitoneal injection of tetanus toxoid mixed with complete Freund’s adjuvant. At 2 weeks interval from the primary immunization, booster doses of the tetanus toxoid mixed with incomplete Freund’s adjuvant were injected in the BALB/C mice. The Freund’s adjuvant was used to induce sustained immune response against the tetanus toxin. The last booster dose was given in a form of purified tetanus toxin 4 days before fusion. This immunization protocol was similar with those applied by Hunt and Zola (2000) and Ausubel and Brent, (2003). This immunization procedure, however, differs from that applied by Kenimer and William (1983) who used diphtheria tetanus- pertusis vaccine (DPT) for S/C primary immunization of BALB/C mice followed by intraperitoneal booster injections of purified tetanus toxoid only. Sheppard and Deborah, (1983) on the other hand immunized BALB/c mice intraperitonealy with tetanus toxoid without using of adjuvant for preparation of monoclonal antibodies against tetanus toxin.
To monitor the progress of immunization procedure, an ELISA screening test was used for declaration of the immunization level and selection of well responder (immunized) BALB/C mice. This technique is nearly the master screening technique used in monoclonal antibody technology because of its the rapidity and the easiness with which the test can be done with several hundred of samples (Davis et al.,1983, Loeb and Quimby, 1999, Kane and Banks, 2000). Also, Farzad and James (1986) reported that ELISA is a rapid and sensitive immunoassay for the quantitation of anti-tetanus antibodies.
The present study revealed, before fusion procedure the tissue culture plates were supplemented with peritoneal macrophages as feeder cells, which support the growth of the hybridoma. This goes hand to hand with that done by (Astaldi and Janssen, 1980, Schidegger and Groth, 1980, Mao and France, 1984).
Although other types of feeder cells can be used, e.g. thymocytes (Davis et al., 1983), macrophages as feeder cells probably secrete growth factors, which stimulate the growth of the hybridomas, remove toxic by-product from media and exert a synergistic effect. Also it increase markedly the yield of hybrids and remove dead parental cells and debris from the cultures (Schidegger and Groth, 1980, Davis et al., 1983, Mao and France, 1984, Mike Clark, 1995 and Quinlan and Kennedy, 1994).
In concern with the fusion procedure, the splenocytes (B-cells) harvested from a hyper-immunized BALB/C mice were fused with myeloma cell lines (P3NS1). The myeloma cells were selected in log phase with viability over 90%. The fusion between B-cells and myeloma cells was supported by the fusogenic material called polyethylene glycol (PEG) 1500, 40% concentration. PEG is a polywax material that promotes cell adherence and exchange of their nuclei. PEG induces the formation of somatic cell hybrids and facilitated an improved and simplified technique for cell fusion in vitro (Davidson and Gerald, 1976). Normally cell fusion can occur spontaneously in culture at low levels but its incidence can be increased by treatment of cells with a fusogenic agent like PEG (Caroline Macdonald, 1998, Hunt and Zola, 2000, Little and Gall, 2000 and Zola and Holbrook 2002). This agent still is widely used in infusion technology by most laboratories at a concentration of 40-50% (W/V). Schidegger and Groth (1980) and Davis et al. (1983) reported that fusion can be performed with several types of PEG (1000 to 4000 MW) at concentration of 45 – 50 % and gave the best results.
In fusion technology different ratios between myeloma cells and splenocytes have been tried by several authors. In the present work 1:10 cell ratio between myloma and spleenocytes has been used. This is similar to what was done by Kenimer and William (1983) who fused (108) spleen cells and (107) myeloma cells in fusion process in the presence of 50% PEG 1000 solution added to the mixture of both cells. On the other hand, Sheppard and Deborah (1983) fused 2×108 immunized rat spleen cells with 108 rat myeloma cells with good fusion results.
The produced spleen-myeloma cells hybridomas, which were selected from the unfused cells and undesirable fused cells, using hypoxanthine aminopterin thymidine (HAT selective medium) according to Kohler and Milstein (1976); Schidegger and Groth (1980) and Little and Gall, (2000). Exposure of fusion cultures to a selective medium with hypoxanthine, aminopterine and thymidine reduced the labor involved and increased the yield.
Cell fusion is random therefore; the cell culture contains a mixture of myeloma-spleen cells fusion, myeloma-myeloma cells fusion, and spleen-spleen cells fusion. Selection of myeloma- spleen cells fusion can be only accomplished by culturing the cell mixture in hypoxanthine aminopetrin thymidine (HAT) medium. Plasmacytoma cell lines are deficient in enzyme hypoxanthine guanine phosphoribosyl transferase (HGPRT) responsible for incorporation of hypoxanthine.
Post fusion care started seven days after plating out the cells in HAT medium with replacement of half the medium with fresh medium containing HT, instead of HAT. At this time, small hybridoma growth was detected at the margin of several wells. Supernatants from these wells were collected and tested for the presence of Mabs using ELISA according to Hunt and Zola, (2000). Our findings, 12 days post fusion, hybridoma cell growth that covers 10% – 50% of the surface area of the wells were screened for antibody production against tetanus toxin using ELISA technique. Most of researchers use ELISA for screening of monoclonal antibody production by hybridoma (Kenimer and William, 1983; Volk and Bizzini, 1984; Hunt and Zola, 2000; Legert, 2000; Kane and Banks, 2000).
In the present work we succeeded to recover 10 positive hybridoma producing specific monoclonal antibodies against tetanus toxin. Five out of these hybridoma cell lines were cloned successfully. Cloning of the positive hybridoma was done using limiting dilution technique to isolate a single stable clones producing of specific monoclonal antibodies against tetanus toxin. Cloning was repeated three times in order to remove the non-producing cells from the clones and to avoid the overlapping of the antibody producing cells by the non-producer ones. Also to guarantee the monoclonality of the antibody producing cell (i.e. to confirm its derivation from a single hybrid cell). This agrees with that stated by Kennett, (1979); Kennett, (1980); Sheppard and Deborah, (1983); Mike Clark, (1995) and Little and Gall, (2000).
Furthermore, to evaluate the biological activity of produced monoclonal antibodies by using tetanus toxin neutralizing test in mice, five clones proves neutralizing potential and gave a 100% survival rate in the tested mice. These findings coincide with those reported by Mizugguchi and Yoshida, (1982), Kenimer and William, (1983), Sheppard and Deborah, (1983), Volk and Bizzini, (1984) and Shang and Yeh, (1988). These authors used tetanus toxin neutralization assay in mice to determine the biological activity of the produced monoclonal antibodies from hybridomas culture and ascitic fluid against tetanus toxin.
Today the industry is working as furiously as ever to perfect the design and production of Mabs for therapeutic purposes and more than 200 Mabs types are now in clinical trials. One of the known examples of monoclonal antibodies that have been approved for human use is the therapeutic drug PALIVRMAB used to cure severe respiratory illness in newborn infants infected with syntial virus. Another example is CAMPATH1 used to prevent host versus graft disease and graft rejection in patients receiving bone marrow transplants and is licensed now in treating leukemia. Similarly we aim by the present work to begin the preliminary step required for production of anti-tetanic serum using monoclonal antibody technology. We are sure that more intensive and hard work is required to reach to this phase but at least the five obtained hybridoma cell lines will be further intensively characterized and reevaluated for the achievement of our main goal.
In conclusion, the five hybridoma clones namely (Te-D11H6, Te-D11D8, Te-F2F11, Te-G9C11 and Te-B3C9) had been identified and all were found to belong to IgM isotype by using agar gel procedure (Ouchterlony double Diffusion) and mouse immunoglobulin isotyping kits. On the other hand, Kenimer and William, (1983) were assayed the clones supernatant for their immunoglobulin class and subclass specificity by ELISA. Also, Volk and Bizzini, (1984) were determined the antibody isotypes on culture supernatant by using an ELISA reaction with a mouse immunoglobulin subtype identification kit.
References
- Ali. M. (2001): Monoclonal antibodies production against breast cancer. Ph. D. Thesis, Military Medical Academy
- Astaldi, G.C.B.and Janssen, M.C., (1980): Human endothelial culture supernatant (HECS): growth factors for hybridomas. J. Immunological method, 125, 1411.
- Ausubel F. M and R. Brent (2003): current protocols in molecular. Biology. John Wiley & Sons, New York.
- Buxton, A. and Fraser, G. (1977): Clostridia. Animal Microbiology, 1st Ed. J.B. Lippinocott Company, USA. pp 205-227.
- Caroline, Macdonald. (1998): Primary Culture and the establishment of cell lines .PP:149-178.Cited from Basic cell culture. A practical approach. Edited by J.M.Davis. Oxoford university press.
- Carter, G.R. (a) (1973): Clostridia. Outline of veterinary bacteriology and Mycology, 2nd Ed., Boston oxford London Edinburgh, Melbourne.
- Carter, G.R. (b) (1973): Diagnostic procedures in veterinary medicine 2nd Ed, Charles C. Thomas publisher, USA.
- Davidson R Land Gerald P.S (1976): Improved techniques for the induction of mammalian cell hybridization by PEG. Somatic cell genetics, Vol .2, 2, 165 – 176.
- Davis William C.; Mcguire, T.C and Travis C. (1983): Biomedical and biological application of monoclonal antibody technology in developing countries. Periodicum biologorum, Vol, 85, No 3, 25 9-282.
- Farzad Z. and James K. (1986): Measurement of human and mouse anti-tetanus antibodies and isotype analysis by ELISA. Journal of Immunological Methods (1986), 87(1): PP 119-125.
- Ganong, W.F. (1981): Review of medical physiology. 10th edition, Lange medical publications drawer L., Los, Atlas, California.
- Hay Frank C. and Westwood Olwyn M.R. (2002): Practical immunology. Monoclonal antibodies: production, purification and enzymatic fragmentation.PP:40-70. 4th, Black well science.
- Hunt B. and Zola H. (2000): Hybridoma technology making monoclonal antibodies. PP: 17-42. Cited from Monoclonal antibodies, preparation and use of monoclonal antibodies and engineered antibody derivatives. Edited by Heddy Zola.
- Kane M. and Banks J.N. (2000): Raising antibodies. Cited from Immunoassays. A practical approach. Edited by J.P.Gosling, Department of biochemistry and national diagnostic center, national university of Ireland galls way (Oxford University). PP:19-58
- Kenimer James G. and William H. (1983): Monoclonal antibodies as Probes of Tetanus Toxin Structure and Function. Infection and Immunity, 1983, Vol. 42, No. 3. p. 942-948.
- Kennett R.H. (1979): Cell fusion, J.Methods Enzymol.1979; 58:345-59.
- Kennett R.H. and Mckearn T.J. (1980): Monoclonal antibodies. Plenum, New York.
- G.Kohler and C.Milstein (1975): Continuous cultures of fused cells secreting antibody of predefined specificity. 1975 August Nature 256, 495 – 497.
- G.Kohler and C.Milstein (a) (1976): Fusion between immunoglobulin secreting and non secreting myeloma cell lines, Eur.J.immunol. Apr; 6(4): 292-5.
- G.Kohler and C.Milstein (b) (1976): Derivation of specific antibody producing tissue culture and tumor lines by cell fusion. Eur.J.immunol. 6, 511-519.
- Legert Klaus D.E. (2000): Immunology, understanding immune system. Micro biology & Immunology Department of biology Virginia polytechnic institute and state university Blacsburg, Virginia. PP: 71-73
- Loeb, W.F. and Quimby. (1999): The clinical chemistry of laboratory Animals, 2nd edition, Philadelphia, P.A: Tayelor and Francis.
- Little M. and Gall F. Le. (2000): of mice and men: hybridoma and recombinant antibodies .Immunology today, Vol. 21, No.8 PP: 355-412.
- Mao, S.J.T. and France, D.S. (1984): Enhancement of limiting dilution in cloning mouse myeloma- spleen hybrido mas by human low density lipoproteins. Immunological method, 75,309-310.
- Mike Clark. (1995): Monoclonal Antibodies: principles and applications. Editors; J.R. Birch and E. S. Lennox. Copyright © 1995 wiley-liss, Inc. PP: 1-45.
- Mizugguchi J. and Yoshida T. (1982): Requirement of at least two distinct monoclonal antibodies for efficient neutralization of tetanus toxin in vivo. Naturwissenschaften (1982), 69(12): PP 597-598.
- Pope, C.G and Porter, R.R. (1963): British Medical Bulletin 19:197-230.
- Quinlan and Kennedy. (1994): Enhanced cloning efficiencies of murine hybridomas using human plasma supplemented medium. J. Immunological method, 123: 157-165.
- Schidegger D and DE Groth S.F. (1980): Production of monoclonal antibodies: strategy and tacties. J. Immunological method. 35(1-2):1-21. (1980).
- Shang H.F. and Yeh M.Y. (1988): Protective murine monoclonal antibodies to tetanus toxin. J. of Clin. Investigation, 1988 Nov; 21(4): 199-209.
- Sheppard Anthony J. and Deborah Cussell. (1984): Production and Characterization of Monoclonal Antibodies to Tetanus Toxin. Infection and Immunity, 1984, Vol. 43, No. 2. p. 710-714.
- Stone M.J. (2001): Monoclonal antibodies in the prehybridoma: a brief historical perspective and personal reminiscence. Clin Lymphoma, 2001 Dec; 2(3):148-54.
- Volk W.A. and Bizzini B. (1984): Neutralization of tetanus toxin by distinct monoclonal antibodies binding to multiple epitopes on the toxin molecule. Infection and Immunity, .P.P: 604-609.
- Zola H. and Holbrook FL. (2002): Tolerization as a tool for generating novel monoclonal antibodies. Immunol cell Biol. Aug; 80(4): 319-22.