Information
Journal Policies
PD-1/PD-L1 Checkpoint in Lymphoprolipherative Malignancies: Focus on Critical Points for Tissue Assessment
Antonella Bianchi1,Ombretta Annibali2,Alba Grifoni3,Giuseppe Avvisati4,Anna Crescenzi5
2.Unit of Hematology, Stem Cell Transplantation, Transfusion Medicine and Cellular Therapy, University Hospital Campus Bio-Medico, Rome, Italy.
3.Division of Vaccine Discovery, La Jolla Institute for Allergy and Immunology, La Jolla, CA, USA.
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
PD-1/PD-L1 checkpoint has an important role into the control of the immune responses and it is frequently used as a mechanism of immune escaping by neoplastic diseases. Beside its important role in solid tumors, evidences in hematological malignancies are emerging. This review summarizes the most recent data highlighting the role of immunohistochemistry for the assessment of PD-1/PD-L1 checkpoint in lymphoproliferative disorders. In this setting, various critical issues such as the choice of the antibody, the protocol for staining, the staining pattern and the cut-off for interpretation, need to be standardized. Moreover, pre-analytical factors also may generate false positive or negative results. Herein we analyzed the factors that influence PD-L1 immunohistochemical assessment when used as a predictive biomarker.
PD-1, PD-L1, PD-1/PD-L1 checkpoint, lymphoproliferative disorders, hematologic malignancies, immunohistochemistry,Hematology
1. Mechanism Of Pd-1/Pd-L1 Checkpoint And Its Downstream Effects
Programmed cell death protein 1 (PD-1) is a transmembrane receptor, expressed on activated CD4+ and CD8+ T-cells, B-cells, natural killer (NK) cells, macrophages and dendritic cells. PD-1 is not constitutively present on resting lymphoid cells but its expression can be induced after stimulation and prolonged inflammation. PD-1 activation after binding with its ligands induce downregulation of T-cell function by suppressing signaling pathways downstream of T-cell receptor (TCR) stimulation. PD-1 has two main ligands, programmed cell death protein 1 ligand 1 (PD-L1) (B7-H1 or CD274) and PD-L2 (B7-H3 or CD273), both of which are transmembrane proteins expressed on the membrane surface of several immune cell population. PD-L1 is expressed constitutively on T-cells, B-cells, dendritic cells and CD4+CD25+ regulatory T-cells (Treg), while its expression on epithelial cells and other non-hematopoietic cells is induced by Interferon-ɣ release. PD-L2 is mainly expressed on antigen presenting cells such as macrophages and dendritic cells and it is induced by stimulation with Interferon-ɣ and Interleukin-4. PDL1/PDL2 checkpoint provides a regulatory feedback mechanism to limit the effector phase of T-cell expansion and function inducing T cell exhaustion and therefore playing an important role in the immunological tolerance[1].
There is a growing scientific evidence of PD-L1/PD-L2 expression on the neoplastic cells of many different cancers[2]. By binding to PD- 1 on T-cells leading to its inhibition, PD-L1 expression is a major mechanism of immune-escape exerted by tumour cells.
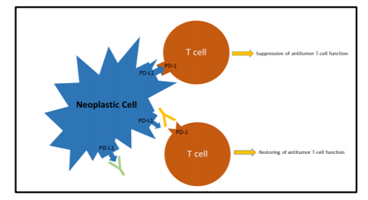
Two different mechanisms may be responsible for PD-L1 over-expression: a) constitutive expression by tumour cells due to genetic alterations of the PD-L1 and PD-L2 locus of chromosome 9p24.1 (gains, amplifications or fusions), or b) induced expression in response to specific cytokines, particularly Interferon-ɣ, released by the anti-tumour immune response (adaptive immune resistance).[1] The disruption of PD-1/PD-L1 interaction using antibodies directed against PD-1 (Nivolumab and Pembrolizumab) or PD-L1 (Atezolizumab) restores the T cell capability to exert cytolytic functions directed to neoplastic cells (Figure 1).
2. Clinical Use Of Pd-1/Pd-L1 Checkpoint
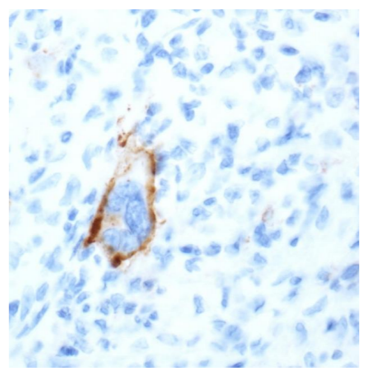
The success of checkpoint blockade therapy in the treatment of different solid tumours encourages the research for similar results in the wide spectrum of lymphoproliferative diseases. Classical Hodgkin‟s Lymphoma (cHL) is the prototype of lymphoid malignancies susceptible to blockade of PD-1/PD-L1 checkpoint. In the phase I study of Ansell et al., in 23 patients, with relapsed or refractory cHL, treated with Nivolumab, all Reed–Sternberg cells, which were identified by their characteristic morphologic features and staining for PAX5, expressed PD-L1 protein (Figure 2) [3]. The overexpression is associated with the amplification of 9p24.1 frequently demonstrated in Reed–Sternberg cells. Given the excellent response rates and acceptable side effect profile, demonstrated in a pivotal phase II trial, the US Food and Drug Administration approved in May 2016 Nivolumab for the treatment of cHL that has relapsed or progressed after autologous haematopoietic stem cell transplantation and post-transplantation Brentuximab Vedotin (BV) [4].
Numerous clinical trials are underway involving anti-PD-1 or anti-PD-L1 antibodies as single therapies or in combination with other therapies in lymphoid malignancies. [5] As PD-L1 protein can be detected by immunohistochemistry (IHC) on Formalin-Fixed and Paraffin-Embedded tissue sections, several issues have been addressed to such activity in order to standardize the procedure and the scoring system [5-8].
3. Benefits And Pitfalls Of Immunohistochemistry
The assessment of PD-L1 expression through immunohistochemical staining is advocated as the finest biomarker that allows better selection of patients who are likely to respond to targeted immunotherapy, to improve treatment efficacy and to manage cost of therapies.
However, therapeutic targeting of PD-L1 faces many problems as its non-uniformity in different parts of the tumour and in different sites, its dynamic variability with time, and its intracellular expression (therapeutically not relevant) that must be distinguished from its surface expression (therapeutically relevant). Finally, the expression of PD-L1 in the tumour microenvironment (myeloid cells, antigen-presenting cells) might also exert an unpredictable influence [9].
Moreover, as the validation of standardized procedures for assessing PD-L1 expression in the wide spectrum of lymphoproliferative neoplasms is paramount, but currently missing, the pathologist should deal with various critical issues as the choice of the antibody, the protocol for staining, the staining pattern and the cut-off for interpretation.
4. Selection Of The Primary Antibody
Immunohistochemistry testing is relatively inexpensive and easy to perform, but interpretation is complicated by the fact that different clones of monoclonal antibodies raised against the same protein are specific for different parts (epitopes) of the PD-L1 molecule. Thus, different clones may give different staining results. A number of PD-L1 antibody clones are available as pre-packaged kits or as free antibodies, using different staining platforms and different staining protocols, with or without enhancement systems. It is possible to produce very reliable and accurate „„home brew‟‟ laboratory-developed tests, however, not every laboratory has the time or experience to validate laboratory-developed tests. For this reason, many laboratories choose to apply ready-to-use (RTU) antibodies and RTU IHC systems.
5. Different Immunohistochemical Procedures And Detection Thresholds: An Update Of The Literature
Among the wide spectrum of lymphoproliferative malignancies, Menter et al. conducted the most exhaustive study about the immunohistochemical assessment of PD-L1 expression. They evaluated the diagnostic and prognostic value of PD-L1on tissue microa on tissue microarrays and whole slides in a total number of 899 cases of cHL and different B-cell lymphoma entities with two different antibodies: clone E1L3N [Cell Signalling, Danvers, MA] and clone SP142 [Roche/Ventana, Rotkreuz, Switzerland].The staining pattern of both antibodies was membranous/sub membranous with occasional dots. Sinus-lining cells and macrophages were considered as positive control. For treatment purpose, only expression of PD-L1 on tumour cells was considered and PD-L1 positivity was defined as at least 5% positively staining tumour cells. To be considered evaluable, cases, especially tissue microarray cases, had to exhibit at least 25% of tissue available for morphologic analysis and at least 1 positively staining tumour-infiltrating macrophage as positive internal control. They had to contain unequivocal tumour cells, and, in cases of cHL, at least 5 Hodgkin and Reed-Sternberg cells or, in cases of Nodular Lymphocyte-Predominant Hodgkin‟s Lymphoma, 4 lymphocytic & histiocytic cells[10].
Even according to Menter, in cases of Burkitt Lymphoma, cHL, Diffuse Large B Cell Lymphoma (DLBCL), Marginal Zone Lymphoma with a high-grade component, Nodular Lymphocyte-Predominant Hodgkin Lymphoma, and Primary Mediastinal B Cell Lymphoma (PMBL), tumour cells were morphologically easily identifiable for scoring. In the so-called low-grade B cell lymphomas, only tumour cell−rich areas, as assessed on consecutive slides stained with tumour cell typical markers such as Cyclin D1, CD20, or bcl6, were considered for PD-L1 evaluation. PD-L1 expression was observed in most cases in Hodgkin and Reed Sternberg cells of cHL and on almost all tumour cells; in 31% of DLBCL cases on a considerable amount of tumor cells; in 35% of PMBL; in 5% of Follicular Lymphoma (FL) only on a small proportion of tumor cells; in 10% of Marginal Zone Lymphoma with a high-grade component. All cases of Lymphoplasmacytic Lymphoma, Mantle Cell Lymphoma, Burkitt Lymphoma and T-cell and histiocyte-rich B-cell Lymphoma were negative for PD-L1 on tumour cells, but the latter showed PD-L1 expression on infiltrating cells [10].
Bledsoe et al. performed immunohistochemistry for PD-L1 on tissue microarray and on whole-tissue sections of a total of 49 cases of PMBL stained with intra-laboratory validated antibodies, using an automated immunostainer (BOND-III, Leica Biosystems, Buffalo Grove, IL). For all antibodies scores were assigned for staining intensity (0: no staining, 1+: weak staining, 2+: moderate staining, 3+: strong staining) and percentage of lymphoma cells staining (0: 0%, 1+: 1–25%, 2+: 26–50%, 3+: 51–75%, 4+: >75%). Staining intensity and frequency scores were multiplied to yield an overall H-score ranging from 0 to 12, which was categorized as follows: no staining (0), weak/focal or low overall staining [1–3] and moderate/strong/diffuse or high overall staining [4–12]. The immunohistochemical stains were evaluated independently by two pathologists who were blinded to patient outcome, and the interobserver agreement for antibody staining was overall high. PD-L1 was expressed by tumor cells in 71% of cases of PMBL. On univariate analysis, high overall PD-L1 staining (H-score 4–12) was associated with longer progression-free survival[11].
PD-L1 expression assessment was performed by Vranic et al. in refractory and/or resistant lymphomas of B- and T-cell lineages. FFPE sections were treated with PD-L1 Antibodies (Clones: SP142 and SP263, Ventana Medical Systems, Tucson, AZ) using automated immunohistochemical methods at a CLIA/ CAP/ISO15189-certified lab (Caris Life Sciences, Phoenix, AZ). Cases were considered positive when 5% of the neoplastic cells exhibited membranous positivity with 2+/3+ intensity. PD-L1 expression was also evaluated in adjacent population of reactive/inflammatory cells. Overall PD-L1 positivity (>5% positive cancer cells with 2+/3+ intensity) was 42% by SP142 and 46% by SP263 antibody. The highest PD-L1 positivity was confirmed in cHL (91%) and PMBL (100%). DLBCL were frequently positive (50%) irrespective of subtype. Follicular, Peripheral T-cell and Mantle cell Lymphomas were rarely positive, while Small Lymphocytic Lymphoma/Chronic Lymphocytic Leukemia (CLL) and Marginal Zone Lymphoma were consistently negative. A single case of DLBCL arising in FL exhibited positivity exclusively in large cell component (~25% by 2–3+ intensity by both antibodies) while low grade component was devoid of PD-L1 expression. The mean percentage of PD-L1 positivity in tumor cells was 21% (range, 5– 100%). The Authors also evaluated PD-L1 positivity in adjacent inflammatory (reactive) cell population; fifty-five out of 58 cases (95%) had PD-L1 positivity in reactive/inflammatory cells (ranging from single cells to abundant reactive population as typically seen in cHL); only 3 cases had no visible PD-L1+ reactive cells by both clones[12].
Berghoff et al. performed immunohistochemistry for PD-L1 with anti-human monoclonal mouse antibody (5H1, provided by Dr. Lieping Chen, Yale University, dilution 1 : 100) on tissue specimens of 20 immunocompetent patients with Primary Central Nervous System Lymphomas, treated with neurosurgical resection. A specific, strong, and fully membranous immune reactivity on more than ~5% of any cell type was considered positive. They observed strong PD-L1 expression on tumour cells in 20% of cases with prominent membranous expression and on intra-tumoral histiocytes in 20% of tumours [13].
Expression of PD-L1 was also demonstrated in advanced stage EBV-associated Extranodal NK/T cell Lymphoma by Kim et al. Immunohistochemistry was performed by using a rabbit anti-PD-L1 (E1L3N) XP® monoclonal antibody (Cell Signalling Technology, Danvers, MA, USA). Expression was semi-quantitatively evaluated in a representative area with >80% of tumour cells. Tumour areas with >20% of necrosis were excluded. The proportion of total PD-L1+ cells including tumour cells and peri-tumoral reactive cells was also scored independently. Immunohistochemical expression of PD-L1 was scored according to the proportion of stained cells and grouped into the following categories: 0, 1–9, 10–24, 25–50, and ≥50 %. Staining intensity was assessed in a three-tiered approach: weak, moderate, or strong. PD-L1 expression of tumor cells was deemed as positive when ≥10% of tumour cells showed moderate or strong immunoreactivity. Tumour microenvironment was considered as positive for PD-L1 if ≥10% of total cells demonstrated moderate or strong staining intensity. The Authors concluded that PD-L1 expression is a favourable prognostic indicator [14].
An exhaustive summary of studies assessing PD-1/PD-L1 protein expression in non-Hodgkin Lymphomas (NHL) and its impact on patient outcome has been recently published by Gravelle et al. The Authors reported that recent preclinical trials with immune checkpoint blockade therapies have shown efficacy, especially in relapsed/refractory (r/r) NHL. For example, 36% of r/r DLBCL patients and 40% of r/r FL displayed a complete response or partial response with Nivolumab. Pembr olizumab had an overall response rate (ORR) of 37.5% in PMBL patients and 21% in CLL patients. On the contrary, Tcell lymphomas patients treated with Nivolumab responded at a lower rate. In addition, the clinical impact of PD-L1 status has not yet been definitely established in EBV+ lymphomas and Anaplastic Lymphoma Kinase-positive Anaplastic Large Cell Lymphoma, both frequently positive for PD-L1[15].
Recently, we Reported expressions of PD-L1 also in the extra-medullary lesions of multiple myeloma, supporting the fact that PD-L1 up-regulation occurs also outside the bone marrow location[16].
6. Role Of Pre-Analytical Factors
Based on the above mentioned evidences, the standardization of IHC is fundamental for the assessment of the expression of PD-L1 when used as a predictive biomarker and needs accuracy during all the histological process. Pre-analytical factors, other than analytical and post-analytical steps, may interfere with immune histochemical results. The pre-analytical stage begins as soon as the tissue sample is removed and the time to fixation is critical. Degeneration is caused primarily by autolysis, a process of self-digestion by enzymes contained within cells and begins immediately. Fixatives are used to stop degeneration while preserving the structure and integrity of the tissue elements. Unfortunately, to date, no single fixative has proven to be ideal for all targets and detection methods. The “gold standard” fixative is 10% Neutral Buffered Formalin with pH 7.0 to 7.4. It has traditionally been used by pathologists, perhaps because the ingredients are relatively inexpensive and the solution is simple to prepare and stable when stored. It is very important that tissue be “properly” fixed and that sufficient time is given to ensure completion of this process. A minimum of 24 hours fixation should be applied to all samples. Alcohol fixation may cause false negative or positive results and should be avoided. Sections for IHC are cut at 3-5 µm. Thicker sections may cause difficulty during staining and problems in interpretation due to the multi-layering of cells.
7. Quality For Microscopic Assessment
Slide evaluation should be performed by a pathologist using a light microscope. The scoring may be achieved using 10-20x objectives and confirmed at 40 x if needed. The staining needs to be repeated if positive control fails to stain, negative control shows any grade of staining, or if staining background, limiting scoring interpretation, is present in the patient‟s slide. Pathologists must distinguish what constitutes true staining or artifact for each particular assay. Moreover, in some lymphoma subtypes, the identification of PD-L1 positive tumor cells may be challenging due to the abundant reactive/inflammatory cells (e.g. peripheral T-cell Lymphoma and cHL).
In the report the pathologist has to indicate: which biomarker test was used, what the actual score assessment was, the number of cells assessed neoplastic or inflammatory, and then to provide some comments regarding other relevant factors.
8. Cytological Samples
Biomarker tests proven in trials are rarely validated for cytological samples. Cytological samples by Fine Needle Aspiration are rarely employed in lymphoproliferative disease, but they may be the only available specimen in effusion lymphomas. As for other pathological evaluations, immunohistochemistry on cytological samples require a cell block preparation to obtain consistent and reliable results.
9. Conclusions
PD-L1 assessment by IHC as a predictive assay for selecting patients for immunotherapy is now a reality and is set to become a crucial step not only in solid tumors but also in lymphoproliferative disorders. All pre-analytical issues must be borne in mind when immunohistochemical tests are planned. A greater quantity of confirmed data is required for the validation of reporting criteria. In the meantime, however, the pathology and hematology communities will have to collaborate in order to standardize the PD-L1 immunohistochemical assays for the patients being considered for these therapies.
References
- West, E.E., et al., PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest, 2013. 123(6): p. 2604-15.
- Hutarew, G., PD-L1 testing, fit for routine evaluation? From a pathologist's point of view. Memo, 2016. 9(4): p. 201-206.
- Ansell, S.M., et al., PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med, 2015. 372(4): p. 311-9.
- Bond, D.A. and L. Alinari, Emerging treatment options for the management of Hodgkin's lymphoma: clinical utility of nivolumab. J Blood Med, 2017. 8: p. 41-54.
- Ok, C.Y. and K.H. Young, Targeting the programmed death-1 pathway in lymphoid neoplasms. Cancer Treat Rev, 2017. 54: p. 99-109.
- Hude, I., et al., The emerging role of immune checkpoint inhibition in malignant lymphoma. Haematologica, 2017. 102(1): p. 30-42.
- Jelinek, T., et al., PD-1/PD-L1 inhibitors in haematological malignancies: update 2017. Immunology, 2017. 152(3): p. 357-371.
- Sholl, L.M., et al., Programmed Death Ligand-1 Immunohistochemistry--A New Challenge for Pathologists: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med, 2016. 140(4): p. 341-4.
- Topalian, S.L., et al., Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer, 2016. 16(5): p. 275-87.
- Menter, T., et al., Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum Pathol, 2016. 54: p. 17-24.
- Bledsoe, J.R., et al., The immunophenotypic spectrum of primary mediastinal large B-cell lymphoma reveals prognostic biomarkers associated with outcome. Am J Hematol, 2016. 91(10): p. E436-41.
- Vranic, S., et al., PD-L1 Status in Refractory Lymphomas. PLoS One, 2016. 11(11): p. e0166266.
- Berghoff, A.S., et al., PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL). Clin Neuropathol, 2014. 33(1): p. 42-9.
- Kim, W.Y., et al., Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch, 2016. 469 (5): p. 581-590.
- Gravelle, P., et al., Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: a summary of immuno histochemical studies. Oncotarget, 2017. 8(27): p. 44960-44975.
- Crescenzi, A., et al., PD-1/PD-L1 expression in extra-medullary lesions of multiple myeloma. Leuk Res, 2016. 49: p. 98-101