Information
Journal Policies
ARC Journal of Gynecology and Obstetrics
Volume-1 Issue-2, 2016
Abstract
Aim: to assess the efficacy and acceptability of short Medroxyprogesterone acetate (MPA) regimen compared to long MPA regimen for treatment of endometrial hyperplasia (EH) without atypia in postmenopausal women.
Methods: a prospective interventional study conducted on 120 postmenopausal women with EH without atypia who were randomly assigned into two groups; 58 patients received short regimen of 15 mg MPA and 62 patients received long regimen of 15 mg MPA. Follow up endometrial biopsy was done after 6 months. Primary outcome measure was regression of hyperplasia. Secondary outcome measures included side effects and patient acceptability.
Results: There was a statistically significant difference between the two groups regarding regression of EH in the long regimen MPA group (p<0.05) and persistence of EH in the short regimen MPA group (p<0.001). No difference between the two groups regarding the progression of EH to atypia, adverse effects and relapse of bleeding (p>0.05). The rate of hysterectomy was significantly higher in the short regimen MPA group (p<0.001). Long MPA regimen was more acceptable by the patients in comparison to short MPA regimen.
Conclusions: long MPA regimen seems more effective and acceptable therapy in comparison to short MPA regimen in postmenopausal patients with EH without atypia. Larger multicenter studies are warranted to confirm or refute these results.
2.KEYWORDS
3.INTRODUCTION
4.MATERIAL AND METHODS
5.OUTCOME MEASURES
6.STATISTICAL ANALYSIS
7.RESULTS
8.REFERENCES
AUTHOR DETAILS
Said Saleh
Department of Obstetrics and Gynecology , Faculty of Medicine, Menoufia University, Menoufia, Egypt
[email protected]
KEYWORDS
endometrial hyperplasia, progestin therapy, medroxyprogesterone acetate, postmenopausal women.
INTRODUCTION
Postmenopausal uterine bleeding is associated with endometrial cancer in about 10-30% of cases (1).
Endometrial hyperplasia (EH), a noninvasive proliferation of the endometrial epithelium, is generally classified as simple or complex, with or without atypia, based on architectural complexity and nuclear cytology and it is a precursor to endometrial carcinoma (2, 3).
Known risk factors for EH are related to an excess of estrogen relative to progesterone; therefore progestin is used to treat endometrial hyperplasia (2, 4).
Treatment options for women who have EH without atypia include cyclic medroxyprogesterone acetate at 10 mg per day for 14 days per month, continuous megestrol (Megace) at 40 mg per day, or the levonorgestrel-releasing intrauterine system (5-7).
Most of previous studies that assess the efficacy of different progestins for the treatment of EH without atypia included both pre-and postmenopausal women.
The aim of this study was to assess the efficacy and acceptability of short Medroxyprogesterone acetate (MPA) regimen compared to long MPA regimen for treatment of endometrial hyperplasia (EH) without atypia in postmenopausal women.
MATERIAL AND METHODS
This was a prospective observational parallel group study conducted on postmenopausal women presenting with postmenopausal bleeding, from May 2012 to May 2015 in the department of Obstetrics & Gynecology, Menoufia University hospital, Menoufia, Egypt.
The respective approvals of the review board and the ethics committee of the Menoufia Faculty of medicine were obtained before commencing the study. The study protocol and its benefits and complications were explained to all participants, and all recruited patients completed and signed the 'informed consent' form.
Eligibility criteria included menopausal status, no prior diagnosis of endometrial carcinoma, an intact uterus, with histologically confirmed endometrial hyperplasia (EH) without atypia, following history taking which included demographic data as age, reproductive history, menopausal duration and concomitant medical history associated with endometrial cancer, such as body mass index, diabetes mellitus and hypertension were recorded. Clinical examination and transvaginal sonography to assess the endometrial thickness followed by endometrial biopsy by hysteroscopic-guided biopsy from the most suspicious lesions. Menopause was defined as spontaneous cessation of menses for 12 months or more.
Women with associated pathology as uterine fibroid, cervical or vaginal pathology, bleeding tendency, chronic liver disease and any contraindications to progestin therapy were excluded from the study.
Based on the 70-90% regression rate of endometrial hyperplasia under progestin therapy from the literature. Accordingly, at alpha = 0.05 , a total sample size of 96 participants (48 participants in each group) was required for the study to have 90% power to detect 10% difference between the two groups regarding the success rate after adding a percentage of 10% for possible drop out cases during the study.
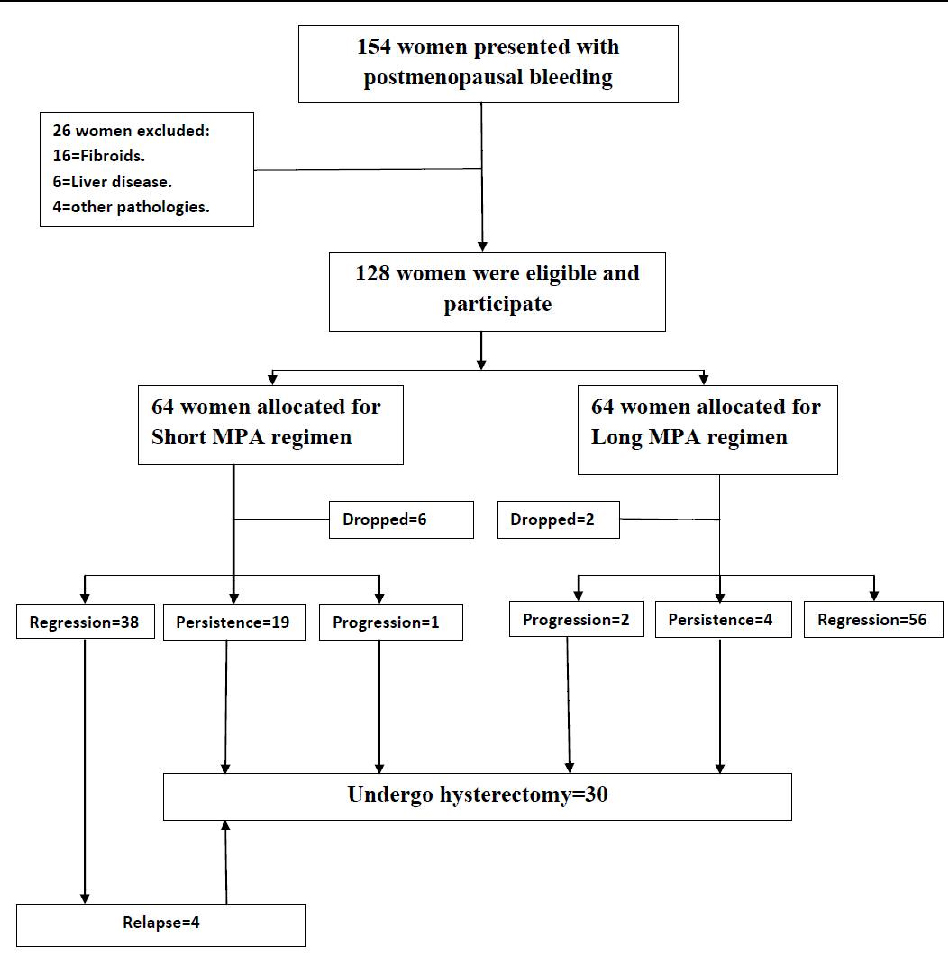
A total of 154 patients were recruited, out of which 26 women were excluded (16 with uterine fibroids, 6 with liver disease and 4 with associated pathologies) , and 128 postmenopausal women (78 with simple EH and 50 with complex EH) were eligible, enrolled and equally allocated into two groups using computer generated simple random tables according to the allocation ratio 1:1 via the use of 128 sequentially numbered, opaque, sealed envelopes. Eight patients were dropped out from the study (stopped treatment and lost follow up), accordingly 120 patients completed the study.
Group 1(Short MPA regimen): included 58 patients (34 with simple EH and 24 with complex EH) who received 15 mg of medroxyprogesterone acetate for 14 days out of 28 days orally (Provera 5mg tablets, Pfizer, USA) for 6 months.
Group 2 (Long MPA regimen): included 62 patients (36 with simple EH and 26 with complex EH) who received 15 mg of medroxyprogesterone acetate per day for 21 days out of 28 days orally (Provera 5mg tablets, Pfizer, USA) for 6 months.
Women were followed via regular visits to the outpatient clinic every 2-4 weeks from the time of hyperplasia diagnosis through a second endometrial biopsy after 6 months and for 18 months at least thereafter. Patients were instructed to report the onset of any adverse effects, specifying the severity, duration and a possible cause-effect relationship with drug administration.
Patients with persistent or recurrent endometrial hyperplasia during the follow-up period were managed by abdominal hysterectomy.
All endometrial specimens were transported in 10% formalin to the pathology laboratory. Microscopic examination was done by two pathologists, individually to reduce observer bias.
OUTCOME MEASURES
Primary outcome measures were regression of hyperplasia after 6 months of progestin therapy. Secondary outcome measures included occurrence of adverse effects during treatment and assessment of patient acceptability. Adverse effects included general adverse effects (headache, nausea & vomiting, mood changes and weight gain) and gynecologic adverse effects (mastalgia, breakthrough bleeding and vaginal discharge). Patient acceptability in terms of overall compliance, overall satisfaction and advisability of the drug to other women.
STATISTICAL ANALYSIS
The data collected were tabulated & analyzed by SPSS (statistical package for the social science software) statistical package version 20(SPSS Inc, Chicago, IL, USA), on personal compatible computer.
Quantitative data were expressed as mean & standard deviation
(X ± SD) and analyzed by applying student t- test for comparison of two groups of normally distributed variables and two groups of not normally distributed variables by applying Mann-Whitney Test.
Qualitative data were expressed as number and percentage (No & %) and analyzed by applying Chisquare test and for 2×2 table and one cell has expected number less than 5 Fisher's exact test was applied.
All these tests were used as tests of significance as follows: P value > 0.05 was considered statistically non significant. P value ≤ 0.05 was considered statistically significant and P value ≤ 0.001 was considered statistically highly significant
RESULTS
Table (1) reveals the patients' characteristics. There was no significant difference between the two groups (p>0.05) regarding age, parity, body mass index, duration of menopause and the presence of associated diabetes mellitus and hypertension.
Table (2) shows the response to progestin therapy and clinical outcome. There was a statistically significant difference between the two groups regarding regression of EH in the long MPA regimen group (p<0.05) and persistence of EH in the short MPA regimen group (p<0.001). No difference between the two groups regarding the progression of EH to atypia and in the relapse of bleeding (p>0.05). The rate of hysterectomy was signifacntly higher in the short MPA regimen (p<0.001).
Table (3) reveals the adverse effects of progestin therapy. There was no significant difference between the two groups (p>0.05) regarding the frequency of general adverse effects (headache, nausea & vomiting, mood changes and weight gain) and gynecologic adverse effects (mastalgia, breakthrough bleeding and vaginal discharge).
Table (4) reveals patient's acceptability. There was a higher overall compliance, overall satisfaction and advisability of the drug to other women in the long MPA regimen (p<0.05).
DISCUSSION
This study included a total of 120 postmenopausal women presented with bleeding and diagnosed as EH without atypia via hysteroscopic-guided biopsy because of the fear of incomplete endometrial sampling upon the use of blind dilatation and curettage or the use of Pipelle sampler.
Because every bleeding in this age group may be caused by endometrial cancer which is the most common cancer of female genital tract worldwide (8, 9). In addition, hysteroscopy allows for an accurate diagnosis in benign endometrial pathology and directed biopsies of suspicious lesions in postmenopausal women (10).
In this study, there was a significant difference between the two groups regarding regression of endometrial hyperplasia (EH) without atypia (65.5% in the short MPA regimen and 90.3% in the long MPA regimen) after 6 months of therapy with more acceptability in the long MPA regimen group.
Regression of endometrial hyperplasia without atypia has been reported with a rate of 60-90% with oral progestin in previous trials (11-13).
The 6 month interval was chosen in this study because it has a better regression rate when compared with withdrawal after 3 months as proved in previous trials (12, 14).
A recent multicenter randomized trial involving 170 women with endometrial hyperplasia without atypia, aged 30–70 years was conducted in Norway. Eligible women were randomly assigned to one of three treatment arms: levonorgestrel-impregnated intrauterine device (LNG-IUS); oral MPA10 mg administered for 10 days per cycle, or continuous oral MPA 10 mg daily, for 6 months. Regressions were obtained for all the women in the LNG-IUS group (53/53) and for 96% of the women in the continuous oral group (46/48). Only 69% of the women in the cyclic oral group were responders (36/52) (15).
The rate of hysterectomy in our series was 25% (30/120) as a result of persistence or progression and recurrence of EH under progestin therapy.
Hormonal therapy resistance has been reported in up to 30% of cases of EH, often attributed to the decreased availability of progestin receptors and alteration of the apoptotic signaling pathway of the endometrial glandular cells (16).
In this study, there was no significant difference between the two groups regarding the frequency of adverse effects. Fortunately, most of these events were little annoying and did not require specific treatment or leaded to stoppage of progestin therapy.
Inability to design a double blind randomized trial and the small number of patients, were the main limitations of this study.
In conclusion, under the conditions of the present study, long MPA regimen seems more effective and acceptable therapy in comparison to short MPA regimen in postmenopausal patients with endometrial hyperplasia without atypia. Larger multicenter studies are warranted to confirm or refute these results.
ACKNOWLEDGEMENTS
The author would like to acknowledge the contribution of the residents and nursing staff of the Gynecology ward and members of Pathology department of Menoufia university Hospital.
REFERENCES
- Izetbegovic S, Stojkanovic G, Ribic N, Mehmedbasic E. Features of postmenopausal uterine haemorrhage. Med Arch. 2013 Dec; 67(6):431-4.
- Reed SD, Newton KM, Garcia RL, et al. Complex hyperplasia with and without atypia: clinical outcomes and implications of progestin therapy. Obstet Gynecol. 2010 Aug; 116(2 Pt 1):365-73.
- Lacey JV Jr. Ioffe OB, Ronnett BM, Rush BB, Richesson DA, Chatterjee N, et al. Endometrial carcinoma risk among women diagnosed with endometrial hyperplasia: the 34-year experience in a large health plan. Br J Cancer 2008; 98:45–53.
- Epplein M, Reed SD, Voigt LF, Newton KM, Holt VL, Weiss NS. Endometrial hyperplasia risk in relation to recent use of oral contraceptives and hormone therapy. Ann Epidemiol 2009; 19:1– 7.
- Hammond R, Johnson J. Endometrial hyperplasia. Current Obstetrics and Gynaecology 2004, 14:99–103.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65: management of endometrial cancer. Obstetrics and Gynecology 2005; 106:413–25.
- Buttini MJ, Jordan SJ, Webb PM.The effect of the levonorgestrel releasing intrauterine system on endometrial hyperplasia: an Australian study and systematic review. Australian and New Zealand Journal of Obstetrics and Gynaecology 2009; 49(3):316-322.
- Korhonen MO. Epidemiological differences between adenocarcinoma and squamous cell carcinoma of the uterine cervix. Gynecologic Oncology. 2004 Dec; 312-317.
- Van Hanegem N. et al. Diagnostic evaluation of the endometrium in postmenopausal bleeing: an evidencebased approach. Maturitas. 2011 Feb; 68(2): 155-164.
- Loiacono RM, Trojano G, Del Gaudio N, at al. Hysteroscopy as a valid tool for endometrial pathology in patients with postmenopausal bleeding or asymptomatic patients with a thickened endometrium: hysteroscopic and histological results. Gynecol Obstet Invest. 2015;79(3):210-6.
- Wang S, Pudney J, Song J, Mor G, Schwartz PE, Zheng W: Mechanisms involved in the evolution of progestin resistance in human endometrial hyperplasia – precursor of endometrial cancer. Gynecol Oncol 2003; 88:108–117.
- Vereide AB, Arnes M, Straume B, Maltau JM, Orbo A. Nuclear morphometric changes and therapy monitoring in patients with endometrial hyperplasia: a study comparing effects of intrauterine levonorgestrel and systemic medroxyprogesterone. Gynecol Oncol 2003; 91:526–33.
- Gallos ID1, Krishan P, Shehmar M, Ganesan R, Gupta JK. LNG-IUS versus oral progestogen treatment for endometrial hyperplasia: a long-term comparative cohort study. Hum Reprod. 2013 Nov; 28(11):2966-71.
- Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol 2012; 125:477–82.
- Orbo A, Vereide A, Arnes M, Pettersen I, Straume B. Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: a national multicentre randomised trial. BJOG 2014; 121(4):477-86.
- Chaudhry P, Asselin E. Resistance to chemotherapy and hormone therapy in endometrial cancer. Endocrine-Related Cancer 2009; 16(2):363–380.