Information
Journal Policies
Periodontal, Microbiological and Immunological Clinical Conditions in a 9-Year-Old Child with Localized Advanced Periodontitis: Case Report in a 18-Month Follow-Up
Bruna Lara Franca1, Amanda Almeida Costa2, Luís Otavio Miranda Cota3, Fernando Oliveira Costa3
2.Master's student in Periodontics, Dentistry School, Federal University of Minas Gerais, Belo Horizonte/Minas Gerais – Brazil.
3.PHD, Professor of Periodontics of the Department of Clinical, Pathology and Dental Surgery, Dentistry School, Federal University of Minas Gerais, Belo Horizonte/Minas Gerais – Brazil.
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The objective of this study is to report a clinical case of a 9 year old child diagnosed with localized advanced and high risk periodontitis through periodontal clinical parameters and radiographic, microbiological and immunological exams. In the clinical examination was observed probing depth alteration of the four first molars, although there was no plaque compatible with the periodontal condition. Radiographic examination revealed angular bone loss in the mesial sites of elements 26, 36 and expressively of 16. Microbiological and immunological tests were performed from the collection of crevicular gingival fluid and saliva. Thus, total bacterial load count and specific bacterial levels of Aggregatibacter actinomycete- mcomitans; Tannerella forsythia, Porphyromonas gingivalis; Treponema denticola; Prevotella intermedia and Actinomyces naeslundii were performed by qPCR (Polymerase Chain Reaction - real time), while levels of the biomarkers Interleukin 10 (IL-10), Interleukin 6 (IL-6), Interleukin beta (IL-β), Metaloproteinase of matrix-8 (MMP-8) and tumor necrosis factor alpha (TNF-α) by ELISA. The proposed treatment included non-surgical and surgical periodontal therapy and antimicrobial use. During the 18 month follow-up of the patient, the mechanical and chemical periodontal treatment promoted a significant improvement in all clinical and radiographic periodontal parameters and positively influenced the patient's bacterial and immunological profile.
Periodontitis, Localized aggressive periodontitis, Bone graft, Antibiotic therapy
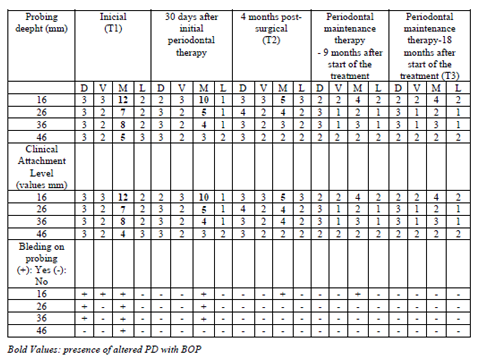
Aggressive periodontitis (AgP); Interleukin 10 (IL-10); Interleukin 6 (IL-6); Interleukin beta (IL-β); Metaloproteinase of matrix-8 (MMP-8); tumor necrosis factor alpha (TNF-α); plaque index (PI); probing depth (PD); bleeding on probing (BOP); clinical attachment level (CAL); (T1) previous to periodontal therapy; (T2) 4 months after treatment; (T3) 18 months after the start of periodontal treatment.
1. Introduction
Periodontitis is defined as an inflammatory disease of the supporting tissues around the teeth, which can cause irreversible loss of the periodontal ligament and alveolar bone, mobility of teeth and, ultimately, if untreated, tooth loss [1]. The disease is caused by an exacerbated immune response to microbial communities resident in the teeth, which extend into the subgingival region. Usually, and for most people, the host lives in symbiosis with this dental biofilm, however, an individual can convert from a symbiotic microbial and immune state to an aggressive and dysbiotic microbiome. These exaggerated inflammatory reactions of the dysbiotic host occur episodically, non-linearly and disproportionately to a diverse collection of risk factors that may result in different degrees of destruction of periodontal tissues [2,3].
The microbial action has, as a response, the generation of inflammatory mediators such as cytokines and prostaglandins, growth factors and lytic enzyme elaboration, in addition to the recruitment of inflammatory cells and activation of osteoclasts, which form the basis of periodontal destruction [4-7].
Aggressive periodontitis (AgP) comprises a group of rapidly progressive forms of periodontitis and with low prevalence (~ 1 to 4%). Besides bacteria, a high level of subject susceptibility must be involved in the expression of disease [2]. In the present study, we report the periodontal clinical, microbiological and immunological profile of a 9-year-old child diagnosed with localized advanced periodontitis in a 18-month follow-up.
2. Materials & Methods
This case report describes the diagnosis and treatment of a female child, caucasian, 9 years old, assisted at the Periodontics clinic of the Dentistry School of the Federal University of Minas Gerais, Belo Horizonte, Brazil, between October 2016 and March 2018. Consent for treatment and disclosure of this clinical case was signed by the child's mother. This case report is described in accordance with the recommendations of the Care Statement cheklist.
The main complaint was pain and mobility in the posterior teeth, in addition to the report of rapid and early loss of deciduous teeth. During the anamnesis the child’s mother reported that at 7 months of age all the deciduous teeth had already erupted and with 5 years old she began to lose them. At 7 years the patient had all the permanent ones, with the second molars erupting and absence of third molars. In addition, there were reports of constant teeth and gum pain, as well as "bad odor and blisters with pus and blood in the mouth", which have been observed since the deciduous dentition. The mother reported that the patient does not have systemic alteration and does not use any type of medication. After complete periodontal and radiographic examinations (described later), the patient received, according to the new classification proposed by American Academy of Periodontology and Federation European the Periodontology [3], the diagnosis of advanced periodontitis (stage IV), localized and high risk (degree C), with a pattern affecting the 4 first molars (previously named as localized AgP).
In the periodontal examination, the following periodontal clinical parameters were recorded: plate index (PI) [8], probing depth (PD), bleeding on probing (BOP) and clinical attachment level (CAL). The PD and CAL were measured circumferentially in all teeth present, and the highest values were recorded for four sites (buccal, lingual, mesial and distal). PB was evaluated at the time of PD measurement or within 30/60 seconds after, and scored at four sites with positive or negative dichotomous values. For all evaluations were used the Hu-Friedy® periodontal probe (UNC-15, Hu-Friedy, Chicago, IL, USA), clinical mirror and gauze. All periodontal examinations were performed by two researchers (BLF and AMC) and always checked by the professor of guidance (FOC). Additionally, in the oral exam there was absence of caries and mucosal lesions. Full-mouth radiographic examinations were performed using the parallelism or long cone technique.
In this case report, periodontal exams were performed at different times during the 18 months of follow-up. However, data referring to periodontal clinical parameters, radiographic examinations and microbiological and immunological collections were reported in 03 times of major clinical importance: (T1) previous to periodontal therapy; (T2) 4 months after treatment and (T3) 18 months after the start of periodontal treatment.
In the mesial, distal, buccal and lingual sites of the four first molars, samples of gingival fluid were collected with the use of absorbent cones in T1, T2 and T3. The methodology of collection and processing was as reported in a previous study [9]. Genomic DNA extraction was performed using the PureLink ™ Genomic DNA Purification Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. The primers employed were designed according to the specific sequence of each microorganism involved (A. actinomycetemcomitans, T. forsythia, P. gingivalis; T. denticola; P. intermedia; and A. naeslundi). The search for the desired target sequences was done by NCBI Nucleotide Search (http://www.ncbi.nlm.nih. gov/). The software Primer 3 (http://frodo.wi. mit.edu/) was used to make the primers and qPCR (Polymerase Chain Reaction - real time) was employed with the Tap Man detection system. The quantification of the microorganisms under analysis was performed by comparing cycle thereshold (Ct).
Unstimulated saliva samples were collected at T1, T2 and T3, whenever possible within two hours after the last meal. The patient was instructed to rinse the mouth with water, and 5 ml of the saliva produced were collected in a Falcon-type millimetric tube and frozen at -80 ° C. The samples were then centrifuged at 3000 rpm for 15 minutes at 4°C, and the supernatant was used to analyze the concentrations of Interleukin 10 (IL-10), Interleukin 6 (IL-6), Interleukin beta (IL-β), Matrix metalloproteinase-8 (MMP-8) and tumor necrosis factor alpha (TNF-α) using commercially available kits (R & D Systems, Minneapolis, MN, USA) and processed by ELISA. The concentrations of the biomarkers were expressed in pg / ml according to the manufacturer's specifications and corrected for saliva (mucin) protein concentration at collection times (T1, T2 and T3).
The Friedman test was used to compare the total bacterial load, the levels of each bacterial species and the biomarkers level in the different phases of the study (T1, T2 and T3). The analyzes were performed using SPSS (Statistical Package for Social Sciences, Version 14.0 for Windows - SPSS Inc., Chicago, IL, USA). The results were considered statistically significant when p < 0.05.
3. Results And Discussion
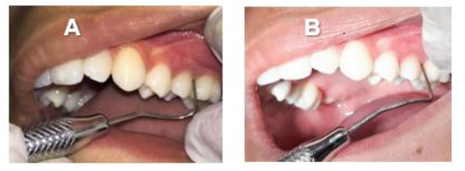
In initial examination (T1), sites with BOP and altered PD were observed in elements 16, 26, 36 and 46 (Table 1) although there was small amount of dental biofilm, not compatible with the condition. The other elements did not present alteration of PD or CAL (values ≤ 3mm). In this exam, 11.5% of the sites had BOP and PI was 33%. It is important to note that there was no alteration in PD or CAL in all incisors. The elements 17, 27, 37 and 47 were not probed since they were at the beginning of the eruption process. No dental elements showed signs of mobility or furcation lesion.
A complete periodontal examination was also performed on the mother and on the 6-year-old patient’s sister, but no site with altered PD (≥4 mm) was found.
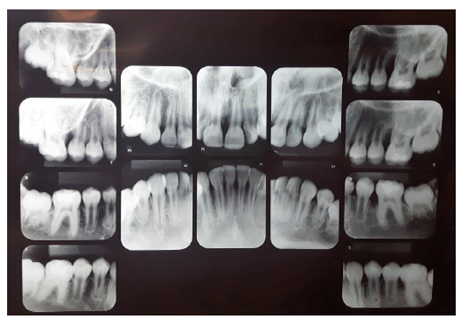
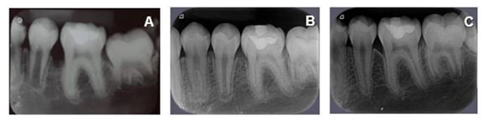
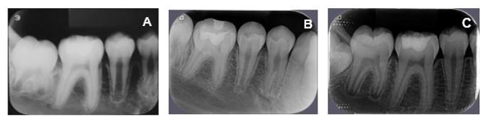
In addition, radiographs of all dental elements were performed (Figure 1). In the periapical radiographs of elements 16, 26 and 36, severe angular bone losses were observed on mesial surfaces.
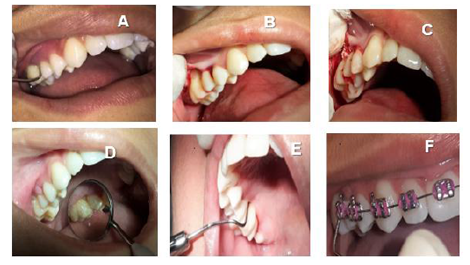
At first consult, it was explained to mother and child the importance of following the instructions of oral hygiene correctly (brushing techniques and use of dental floss). It was also performed coronary polishing and supra and subgingival scaling of elements 16, 26, 36 and 46 under local anesthesia. In addition, use of Amoxicillin (500mg) + Metronidazole (400mg) was prescribed for 15 days [10].
After 15 days, second session was performed with new evidence of plaque, reinforcement on instructions for oral hygiene and coronary polishing. During the first reassessment, 30 days after starting treatment, PI was 22% and mean BOP reduced to 4.2% of the sites. It was observed decrease of PD in all sites, however, with persistence of altered PD in elements 16, 26 and 36. Elements 26 (mesial PD = 5mm with BOP) and 36 (mesial PD = 4mm with BOP) received scaling and root planning under local anesthesia again. However, in mesial of 16, PD, which had the value of 12mm, was now 10mm, still very high (Table 1). Thus, it was decided to perform periodontal surgery involving Kirkland flap procedure and bone graft to fill the bone defect and try to maximize the periodontal repair.
The surgery was performed under local anesthesia with 2% lidocaine with 1: 100,000 epinephrine. During the procedure, a 10 mm intraosseous defect was found in the mesial site of element 16, with preservation of buccal and palatal bone. Scaling and root planning were performed and the bone defect was filled with a mixture of autogenous bone (collected by osteoplasty in the buccal region) associated with the bovine bone graft (Gen Mix, Baumer, SP, Brazil). The suture was made with silk thread (Ethicon, 4.0 Jonhson & Jonhson, SP, Brazil).
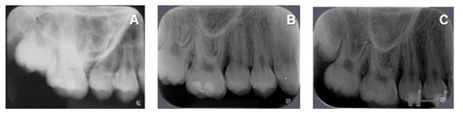
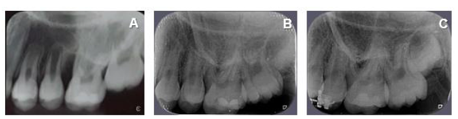
After 45 days of performing these procedures, a significant improvement was observed in the mesial site of element 16 (initial PD = 12 mm for PD = 5 mm) and element 26 (PD = 4 mm with BOP) (Table 1). Thus, despite the significant improvement, as a mild inflammatory condition still persisted, it was decided to reinforce the specific hygiene of these sites, mainly in relation to the use of dental floss and the inclusion of interdental brush, besides keeping the patient in a rigorous program of periodontal maintenance therapy (PMT), performed approximately every 3 months throughout the 18-month follow-up of this case. It should be noted that during PMT at 9 months and 18 months (T3) the PD in the mesial of element 16 and 26 passed to 4 mm without BOP (Table 1). Figures 2, 3, 4, 5, 6 and 7 illustrate the treatment and evolution of this clinical case.
In addition, 12 months after the beginning of therapy, orthodontic therapy was started for correction of left posterior crossbite with anterior crowding.
In relation to laboratory exams, there were significant reductions from T1 to T3 in levels of total and specific bacterial load associated with periodontitis: T. forsythia, P. gingivalis, T. denticola, P. intermedia, A. actinomycetem-comitans (T1 > T2> T3) and also a beneficial increase of A. naeslundii, representative of the blue complex (T1 < T2 < T3).
In addition, the biomarkers Interleukin 10 (IL-10), Interleukin 6 (IL-6), Interleukin beta (IL-β), matrix metalloproteinase-8 (MMP-8) and alpha tumor necrosis factor (TNF-α) were evaluated. It was observed that there was a significant and progressive reduction (T1> T2> T3) in the amount of all pro-inflammatory biomarkers evaluated (Table 2).
During the 1-year and 6-month follow-up, mechanical and chemical periodontal treatment promoted restoration of patient's periodontal health, with a significant improvement of radiographic and clinical aspects. It also positively influenced the patient’s bacterial and immunological profile.
Recently, a new classification proposed at a Workshop of the American Academy of Periodontics and European Federation of Periodontics (FEP) in 2017 brought together the previously named Chronic Periodontitis and Aggressive Periodontitis (AgP) into a single group (Periodontitis) based on the prematurity of the scientific evidence of pathophysiological differences between these two "forms" of periodontitis. According to the new classification of 2017, the diagnosis of the case reported is localized advanced (stage IV) and high-risk (degree C) periodontitis [3].
Once an early and advanced form of periodontitis is installed, several factors may eventually interfere on the progression of inflammation, aggravating it. The lesion’s extent and severity are related to host’s resistance level to microbial factors, since bacteria and their products are able to initiate local responses of the host by generation of inflammatory mediators like cytokines, besides the recruitment of inflammatory cells and activation of osteoclasts, which are the basis of periodontal destruction [6,7,11]. It is noteworthy that the patient in this clinical case presented high levels of proinflammatory cytokines in T1 and, with the success of treatment, there was a significant reduction of all researched biomarkers.
In addition, periodontal diseases are polymicrobial and biofilm-related infections vary widely in microbial composition and diversity between sites and individuals with similar clinical manifestations [12]. They result not only from the presence of specific microorganisms, but from the imbalance between those there are pathogenic and those there are beneficial. To date, studies on the microbiological profile of "AgP" are diversified in their methodology and results [13]. However, according to Faveri et al. [14], bacteria of the species A. actinomycete-mcomitans appear to be associated with the appearance of "AgP" and other bacterial species such as P. gingivalis, T forsythia, Treponema denticola, Campylobacter gracilis, Eubacterium Nodatum, A. naeslundii and P. intermedia, play an important role in progression of the disease. Generally, the amount of A. actinomycetem-comitans found after establishment of the disease is inferior to other species, such as P. gingivalis, which can be explained by the fact that deepening of pockets results in an anaerobic environment, favoring the growth of other pathogens, such as strict anaerobes. In addition, A. actinomycete-mcomitans bacteria are highly virulent and, therefore, even low levels of this species can trigger periodontal destruction, even in non-mature oral biofilm of young individuals. These timely issues have been corroborated by our results that shows a significant reduction of bacteria associated with periodontitis.
It is a consensus that mechanical removal of supra and subgingival dental biofilm by scaling and root planing is the gold standard of periodontal therapy, since it promotes bacterial reduction and often provides resolution for many cases of periodontitis [15, -18]. However, unlike gingivitis and CP, only mechanical therapy does not always provide the expected results when treating "AgP" [15]. In these cases, antimicrobials can be used as adjuvants on treatment to eliminate or reduce the number of specific microorganisms and improve clinical parameters [15,19,20].
Despite controversial results about use and timing of use of antibiotic therapy in treatment of "AgP", in this reported case the antimicrobial treatment was indicated at the beginning of causal therapy. This choice was due to the severity of periodontal destruction in relation to age and was also justified by the literature, which points that immediate application of systemic antibiotics seems to be more advantageous in treatment of "AgP" because it promotes a more rapid and robust reduction of PD [10].
Patients with "AgP" usually have residual vertical bone defects as a result of periodontal disease and regenerative procedures may be one of the possible treatment options. One way of attempting to improve periodontal regeneration is by using bone replacement grafts, as reported in the clinical case of the present study. We believe that filling the bone defect with a mixture of autogenous bone (collected by osteoplasty on the buccal region) associated with bovine bone graft (GenMix, Baumer, SP, Brazil) may have favored periodontal repair and the expressive reduction of PD in element 16 (PD T1=12Mm, T2=10mm e T3= 4mm). GenMix is a composite biomaterial of bovine origin, obtained from inorganic medullary portion, organic cortical portion and a natural binder composed of denatured bone collagen. Currently, this biomaterial is considered a good bone replacement, with reasonable cost and good osteoconductive properties that favor a predictable and efficient bone repair [21].
Given the early onset and rapid progression characteristic of "AgP", early intervention is important. If it is not treated, additional resorption of alveolar bone and tooth loss or extraction will occur sequentially [22], and extensive prosthetic procedures will be required [23]. Furthermore, the treatment should also be directed towards elimination of infection caused by microorganisms. This disease leads to an increase in serum concentration of various inflammatory process indicators, such as chemical mediators and proinflammatory cytokines, as well as presents response of antibodies to different bacteria associated with periodontitis. Therefore, there is an upward concern with the interrelationship between periodontal disease and systemic health, subject of investigations and wide discussion in the dental literature [24].
Currently, the patient is undergoing orthodontic treatment at Dentistry School of UFMG and continues under periodontal maintenance therapy. For movement of teeth, a healthy periodontium is necessary. Thus, in patients with "AgP", orthodontic treatment is only possible when the disease is controlled by careful monitoring before, during and after active therapy and it may be a beneficial adjuvant for providing a good occlusion [25].
4. Conclusion
Clinical, radiographic and laboratory findings of this case report, despite the severity and early onset, showed that the proposed treatment was effective, resulting in periodontal health and control of disease progression.
References
- Costa O Fernando, Vieira Thaís, Cortelli Sheila et al.. Effect of compliance during periodontal maintenance therapy on levels of bacteria associated with periodontitis: A 6-year prospective study. J Periodontol, 2018; 89:519-530.
- Fine Daniel, Patil Amey, Loos Bruno. Classification and diagnosis of aggressive periodontitis. J Clin Periodontol, 2018;45:95-111.
- Tonetti M, Greenwell H, Kornman K. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol, 2018;89:159-172.
- Wahl SM, Costa GL, Mizel DE et al.. Role of transforming growth factor beta in the pathophysiology of chronic inflammation. J Periodontol. 1993;64:7-15.
- Agarwal S, Piesco N, Johns, L. Differential expression of IL-1b, TNF-a, IL-6 and IL-8 in human monocytes in response to lipopolysaccharides from different microbes. JDent Res 1995;74:57-65.
- Alexander D, Martin J, King P et al. Interleukin-1 Beta, Prostaglandin E2, and Immunoglobulin G subclasses in gingival crevicular fluid in patients undergoing periodontal therapy. J Periodontol 1996; 67: 55-62.
- Vucel-Lindberg T, Bage T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev Mol Med., 2013;15.
- Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odont Scan 1964;22:121-135.
- Cortelli JR, Cortelli SC, Jordan S, Haraszthy VI, Zambon JJ. Prevalence of periodontal pathogens in Brazilians with aggressive or chronic periodontitis. J Clin Periodontol 2005;32:860-866.
- Beliveau Dennis, Magnusson I, Bidwell JA, Zapert EF, et al. Benefits of early systemic antibiotics in localized aggressive periodontitis: a retrospective study. J Clin Periodontol 2012;39:1075-1081.
- Madianos P, Bobetsis Y, Kinane D. Generation of inflammatory stimulate how bacteria set up inflammatory responses in the gingival. J Clin Periodontol 2005;32:57-71.
- Socransky S, Haffajee A. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol 1992;63:22-31.
- Feres M, Figueiredo LC, Soares GM, Faveri M.. Systemic antibiotics in the treatment of periodontitis. Periodontol 2000 2015; 67:131-86.
- Faveri M, Figueiredo LC, Duarte PM et al. Microbiological profile of untreated subjects with localized aggressive periodontitis. J Clin Periodontol 2009; 36:739-749.
- Slots, J. Systemic antibiotics in periodontics. J Periodontol, 2004; 75:1553–1565.
- Haffajee, A. Systemic antibiotics: to use or not to use in the treatment of periodontal infections. That is the question. J Clin Periodontol, 2006;33:359–361.
- Heitz-mayfield, L. Systemic antibiotics in periodontal therapy. Australian Dent J, 2009; 54:96-101.
- Herrera D, Alonso B, Leon R, Roldan S, Sanz M. Antimicrobial therapy in periodontitis: the use of systemic antimicrobials against the subgingival biofilm. J Clin Periodontol 2008; 35:45–66.
- Guerrero A, Griffith GS, Nibali L, et al. Adjunctive benefits of systemic amoxicillin and metronidazole in non-surgical treatment of generalized aggressive periodontitis: a randomized placebo-controlled clinical trial. J Clin Periodontol 2005; 32:1096–1107.
- Guzeldemir-Akcakanat E, Gurgan CA. Systemic moxifloxacin vs amoxicillin/metronidazole adjunct to non-surgical treatment in generalized aggressive periodontitis. Med Oral Patol Oral Cirugia Bucal 2015; 20:441-449.
- Manfro R, Nascimento Jr, Loureiro JA, Bortoluzzi MC, D´Campora CR. Successful evaluation of 20 cases of maxillary sinus removal using particulate autogenous bone and inorganic associated Gen-Ox in equal parts (1: 1) - two-year folow-up. ImplantNews, 2009; 6:161-166.
- Costa FO, Cota LO, Costa JE, Pordeus IA. Periodontal disease progression among young subjects with no preventive dental care: a 52-month follow-up study. J Periodontol. 2007; 78:198-203.
- Irokawa, Daisuke et al. Adjunct Antimicrobial Therapy and Periodontal Surgery to Treat Generalized Aggressive Periodontitis: A Case Report. Bulletin Tokyo Dental College, 2016; 57:105-114.
- Vieira AR, Albandar JM. Role of genetic factors in the pathogenesis of aggressive periodontitis. Periodontol 2000; 2014:92-106.
- Gyawali Rajesh, Bhattarai Bhagabat. Orthodontic Management in Aggressive Periodontitis. Int Scholarly Res Notices, 2017:1-8. doi: 10.1155/2017/809815.