Information
Journal Policies
Endovascular Mechanical Embolectomy for Acute Middle Cerebral Artery Occlusion Following Recent Cardiac Surgery
Wilmo C.Orejola MD1*,Ugo Paolucci MD1,Gabriele Di Luozzo MD1,Bruce C.Zablow1,MD Elie M.Elmann MD1
Copyright :© 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Stroke is one of major cardiovascular events that complicate cardiac surgery. Endovascular mechanical embolectomy is reported to restore cerebral blood flow in large intracranial vessel occlusion where thrombolytics failed or were contraindicated. We report application of this new procedure in cardiac surgery. Case #1 is an 82-year-old man in chronic atrial fibrillation who had triple CABG and AVR and developed acute-onset aphasia and right hemiparesis on POD4. CT angiogram showed LMCA M2 occlusion. Endovascular mechanical embolectomy was done and had gradual resolution of neurologic deficits with marked improvement upon discharge and 90-day follow-up. Case #2 is a 55-year-old man who had sextuple CABG and developed chest tightness with ECG changes on POD3. During cardiac catheterization, he had acute-onset aphasia and right hemiplegia. CT angiogram showed LMCA M2/M3 occlusion. Endovascular embolectomy and intraarterial r-tPA were done. He had near complete resolution of aphasia and right hemiplegia upon discharge and 90-day follow-up. Trevo stent retrievers were used within four hours of symptom onset. Endovascular mechanical embolectomy is safe and effective treatment for stroke syndrome following recent cardiac surgery.
Endovascular Procedures, Mechanical Embolectomy, Ischemic Stroke, Cardiac Surgery Complications,Surgery
Ischemic stroke that results in a permanent disability following cardiac surgery is devastating. The current standard medical therapy for acute ischemic stroke is intravenous administration of recombinant tissue plasminogen activator (r-tPA) but its use is limited by narrow therapeutic time window of less than 4.5 hours [1]. It has been also associated with long recanalization times and poor revascularization rates in proximal large arterial occlusions and contraindicated for patients who had recent surgery, or stroke or head injury within the past three months [2,3].
In 2004, the corkscrew-shaped Merci retriever became the first FDA-approved device to be cleared for thrombectomy in acute ischemic stroke [4]. In 2009, the Penumbra system, which places a reperfusion catheter that fragments and aspirates the thrombus, was introduced [5]. Following randomized trials, the Solitaire and Trevo devices, the second-generation, self- expanding stent retrievers recently emerged as the most successful and robust devices for restoration of blood flow to large intracranial vessel occlusion within 8 hours of onset of stroke symptoms and were cleared by FDA in 2012 [6,7]. Most recent AHA/ASA Guidelines for ischemic stroke recommends endovascular treatment with stent retrievers as Class I and Level of Evidence A [8].
We report two cases of endovascular mechanical embolectomy for acute middle cerebral occlusion following recent cardiac surgery using the Trevo stent retrievers.
2. Case Report # 1
The patient is an 82-year-old man with history of type 2 DM, hypertension, dyslipidemia, CVA, chronic atrial fibrillation on Warfarin sodium and a diagnosis of asymptomatic aortic stenosis a year earlier. He presented with shortness of breath and fatigue. He was in Atrial fibrillation. Preoperative carotid Doppler studies were normal.
Transthoracic echocardiogram showed severe aortic stenosis with AVA of 1.0 cm2 and mean aortic valve gradient of 43 mmHg. Left heart catheterization confirmed significantly calcified aortic valve disease and triple vessel coronary artery disease. He underwent aortic valve replacement with bovine pericardial bioprosthesis and triple coronary artery bypass grafting. The procedure was uneventful.
He continued to have rate-controlled atrial fibrillation and was kept on subcutaneous Lovenox 40 mg every 12 hours while optimizing anticoagulation with Warfarin sodium. On POD 4 while his international normalized ratio (INR) was 1.5, he had acute-onset aphasia and right-sided weakness with modified Rankin Scale (mRS) score of 2 and National Institutes of Health Stroke Scale (NIHSS) score of 23.
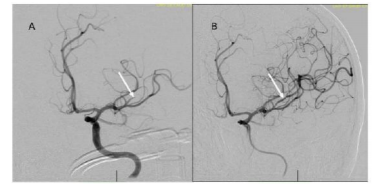
Head CT angiogram showed an old right frontal lobe infarct and an evolving early infarct in the LMCA M2 distribution. Within four hours, emergency transfemoral mechanical embolectomy was done successfully (Figure. 1).
The procedure was started approximately 3 hours and 20 minutes following onset of symptoms. Under general endotracheal anesthesia, through the right femoral artery a Simmons II catheter (Terumo Medical, Somerset, NJ) was used to selectively catheterize the left internal carotid artery (LICA) for digital subtraction mapping. This was exchanged over an Amplatz stiff exchange wire (Boston Scientific, Marlborough, MA) with a 6F 0.088 Neuron Max guide catheter which was advanced into the distal left common carotid artery. A coaxial system consisting of a 5 ACE Max and 3 ACE Max catheters (Penumbra, Inc., Alameda, CA) was positioned in the distal LMCA M1 and the 3 Max Fathom guide wire system was introduced into an occluded LMCA M2/M3 branch supplying the left posterior frontal region. The Fathom wire was removed. A 3 mm x 20 mm Trevo stent retriever (Stryker Global, Kalamazoo, MI) was then introduced and unsheathed. This was left in place for approximately 2 minutes prior to removal of the device using continuous suction applied to the 5 ACE Max catheter. A single pass was made. A control angiogram demonstrated complete revascularization of the occluded left MCA branch with a Thrombolysis in Cerebral Infarction (TICI) score of 3 [9]. No new emboli identified. The sheath was retracted into the ileofemoral artery and an arteriogram was performed. The sheath was removed and the arteriotomy was closed using an 8 Fr. Angioseal (DSM Biomedical, Exton, PA).
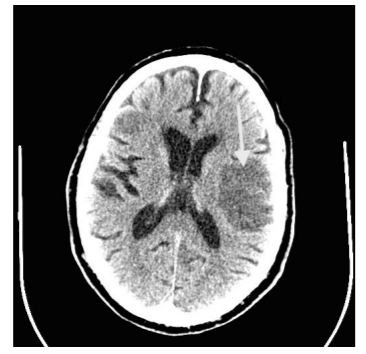
Repeat CT angiogram showed hemorrhagic transformation of the infarct (Figure 2). He had dysphagia and required percutaneous esophagogastric (PEG) access for nutrition. He had gradual resolution of neurologic deficits with near complete resolution of hemiparesis upon discharge to rehabilitation facility on postoperative day 20. A 90- day follow-up estimated mRS at 1 and NIHSS at 15.
3. Case Report # 2
The patient is a 55-year-old man with history of MI, hypertension, dyslipidemia, gastro-esophageal reflux disease (GERD) and a past heavy smoker. He presented with severe angina pectoris and positive stress test. Preoperative carotid Doppler studies indicated less than 50% narrowing of left internal carotid artery origin. Cardiac catheterization confirmed CAD with left main and triple vessel disease.
He underwent sextuple coronary artery bypass grafting. Surgery and immediate postoperative recovery were routine and unremarkable. On POD3 he developed chest tightness with an ECG showing Q-waves in leads v2-v3. Coronary angiogram showed all grafts to be patent and a small non-stentable diagonal branch. During cardiac catheterization, he developed acute-onset aphasia and right hemiplegia with mRS score at 2 and NIHSS score 15. Head CT angiogram showed acute left parietal infarct and LMCA M2/M3 occlusion. Carotid angiogram showed a calcified arteriosclerotic plaque at the origin of the LICA.
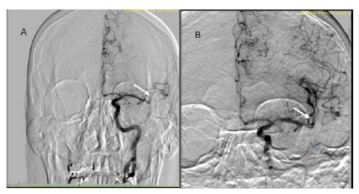
Under conscious sedation, the 6 Fr. short arterial access sheath left in situ from recent cardiac catheterization was exchanged for a new 6 Fr. short access sheath. A 5 Fr. diagnostic catheter maneuvered into the LICA for cranial angiography, which confirmed persistent LMCA M1/M2 occlusion. The diagnostic catheter and sheath were exchanged over the wire for a Neuron 0.088 Max guide catheter, which was positioned in the proximal LICA. A Penumbra 5 Max ACE reperfusion catheter was then advanced into the supraclinoid LICA. A Trevo ProVue 014 microcatheter was maneuvered coaxially over a Synchro 2 micro-guide wire across the area of occlusion in the distal LMCA M1 segment into the M2 superior division. Mechanical thrombectomy was performed with a Trevo 3 mm x 20 mm stent retriever as well as aspiration thrombectomy with the 5 Max ACE catheter during removal of the device. Control angiography revealed modest improvement. Thromb ectomy was then repeated. Control angiography demonstrated near complete revascularization with some distal areas of persistent increased transit time with TICI 2b score. The microcatheter was then maneuvered into the principal M2 superior division for delivery of r-tPA 2mg. Final control angiography showed complete revascularization (Figure. 3). All wires and catheters removed, hemostasis was achieved with a 6/7 Fr. Mynx vascular closure device (Cardinal Health, Dublin, OH). Post-embolectomy magnetic resonance imaging (MRI) of the brain without contrast noted acute cortical based infarction of the LCMA territory without gross hemorrhagic conversion. He had near complete resolution of aphasia and right hemiplegia upon discharge on postoperative day 11. The 90-day follow-up noted mRS score at 1 and NIHSS score 7.
The 2015 updated AHA/ASA Guidelines for ischemic stroke has Class I and Level of Evidence A recommendation for endovascular treatment with a stent retriever if the following criteria are met: modified Rankin Score (m RS) 0 to 1, receiving intravenous r-tPA within 4.5 hours of stroke onset, causative occlusion of ICA or proximal MCA (M1), age 18 years old, NIHSS score of 6, ASPECTS (Alberta Stroke Program Early CT Score) of 6, and treatment can be initiated (groin puncture) within 6 hours of symptom onset. Also to ensure benefit, a reperfusion to TICI grade of 2b/3 should be achieved as soon as possible and within 6 hours of stroke onset [8].
Inadequate data are available to determine the clinical efficacy of endovascular therapy with stent retrievers for those patients with contraindications to receive intravenous r-tPA because of prior stroke, serious head trauma and major surgery like cardiac surgery in the past three months.
The first case we report received successful endovascular treatment with Trevo stent retriever, without intraarterial r-tPA thrombolysis and with TICI flow score of 3. A
hemorrhagic conversion infarct was demonstrated after the procedure, but no progression of neurologic deficits, despite optimizing anticoagulation with Warfarin sodium three days post embolectomy. Whereas, mechanical embolectomy in the second case was done in conjunction with intraarterial r-tPA thrombolysis, despite intravenous r-tPA being contraindicated because of recent cardiac surgery. In both cases neurological status indicated by marked improvement of mRS and NIHSS scores were achieved at discharge and on 90-day follow-up.
This report demonstrates the safety and efficacy of endovascular treatment with stent retrievers for ischemic stroke following recent cardiac surgery. This underscores early recognition of neurologic symptoms by the clinician, diagnostic confirmatory test and quick resolution of thrombus and reperfusion via endovascular mechanical embolectomy or in conjunction with intra-arterial chemical thrombolysis.
Acknowledgement
The authors are grateful for the technical assistance of Peter Torres and Jorge Nuviola for the CT and angiographic images and Deborah Magnan and Ana Rivero-Ortiz for the literature search.
References
- Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379: 2364-2372.
- Ribo M, Alvarez-Sabín J, Montaner J, Romero F, Delgado P, Rubiera M, et al. Temporal profile of recanalization after intravenous tissue plasminogen activator: selecting patients for rescue reperfusion techniques. Stroke. 2006;37:1000-1004.
- Saqqur M, Uchino K, Demchuk AM, et al. CLOTBUST Investigators. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948-954.
- Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Multi MERCI Investigators. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205-1212.
- Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761-2768.
- Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. Trevo versus Merci retrievers for thrombectomy revascularization of large vessel occlusions in ischaemic stroke (TREVO 2): A randomized trial. Lancet. 2012;380:1231-40.
- Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. Solitaire flow restoration device versus Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomized, parallel-group, non-inferiority trial. Lancet. 2012; 380:1241-49.
- Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/ American Stroke Association Focused Update of the 2013 Guidelines for Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment. Stroke. 2015;46:3020-3035.
- Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization Working Group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force. Recommendation on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44:2650-2663.