Information
Journal Policies
Atypical Localizations of Hydatid Disease: US and CT Evaluation
Antonio Gligorievski
Copyright :© 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Hydatid disease (HD) is a unique parasitic disease which is endemic in Macedonia, most frequent localized in liver and lungs. It appears in the form of solitary or multiple cysts, different in size, homogenous, clearly defined, sometimes accompanied by complications.
In this research, we present our experience with atypical localizations of HD: kidney, brain, muscle-skeletal system, spleen, peritoneum, rectum, uterus, ovary, and breast.
The patients were examined by using conventional radiologic and other imaging methods such as ultrasound (US) and computed tomography (CT).
Renal echinococcosis was found in 7 patients, a brain in 3, skeletal in 2, spleen in 3, gallbladder in 2, peritoneal in 3, muscular in 1, ovarian in 2 and breast in one patient.
Native radiograms showed multilayer calcifications and a soft tissue shadow, while contrast radiograms of the abdomen showed signs of intraperitoneal, extraluminal expansion, pressing the surrounding structures. Intravenous urography (IVU) gives pictures of a repressed channel system by a tumor formation, with signs of obstruction and hydronephrosis.
US imaging has a great value in HD screening, being also cheap easily available, simple and with great diagnostic accuracy. CT imaging gives precise topographic and morphologic characteristics of the pathological process, highly sensitive, specific and also accurate.
We can conclude that imaging methods have a great diagnostic value in HD with atypical localization.
echinococcosis, hydatid disease, hydatid cyst, atypical localisations, US and CT
1. Introduction
Hydatid disease (HD) is an endemic disease in the Republic of Macedonia, occurs after contact of the person with an infected dog who is a primary host of hydatid disease or after the use of food contaminated with canine feces, which contains eggs from the echinococcus. [1-3] The infection can be asymptomatic or severe, causing extensive organ damage.
After ingestion, the eggs are released from their casing and the embryo penetrates the mucosa from the duodenum and through the v. Portae comes to the liver, where the embryos are stopped and slowly transforms into a larva of the vesicular type - hydatid cyst (HC). [1,2] The most commonly affected organ is liver (70%), followed by lung (20%) and other organs (10%) such as the peritoneal cavity, spleen, kidneys, brain, bones, genitals, muscles, etc. [4-9]
Hydatid cyst disease, also known as Echinococcosis, is a zoonotic infection caused by the genus Echinococcus. [1-3] Humans are an accidental intermediate host. The outer cyst shell consists of concentric lamellar membranes - a hydatid membrane, and under it is an inner germinal layer of cells. HC is fulfilled with fluid and numerous small cysts that are created from the germinal layer by the swelling and released into the interior of the HC. Their number can reach up to several hundred thousand. In some organs, HC can be surrounded by fibrous tissue - pericitis or adventitia. [1-3]
Echinococcus granulosus forms a large HC, which, by dissemination, produces secondary cysts and Echinococcus multilocularis seu alveolaris, which creates a spongy mass that constantly grows, there are many cysts separated by fibrous tissue in its interior.[1-3]
HC growth is usually asymptomatic and quite slow, the clinical manifestations come from the compression of the affected organs. The process can mimic benign or malignant tumor, single or multiple metastases, abscess, cysts, empyema, and other lesions. [4]
Their recognition is not so simple that clinical and radiological investigations play an important role in the diagnosis of this parasitic disease. [4,10,11,12]
We present our experience with atypical sites of hydatidosis and diagnostic evaluation.
2. Material And Methods
Out of the total number of patients 24, men were 14, and women were 10, aged 5-63 years. Conventional radiological methods, as well as imaging methods, have been used in all patients. Native radiograms of the lung, skeleton, abdomen, urinary tract and intravenous urography (IVU), barium meal (BM) and barium enema (BE) are made.
IVU was performed in 7 patients, after standard preparation of patients with i.v. application of 30-60 ml. 60% iodine contrast agent, making more radiographs at different time intervals.
Small bowel follow-through (SBFT) and BE were applied in 3 patients, after standard preparation, with the use of standard techniques and procedures, several radiographs were made at different time intervals and in several positions and directions.
Ultrasonography examination (US) of the abdomen, retroperitoneum, and pelvis was performed on Toshiba device, using a 3.75 MHz convex probe, and soft tissues US examination was made using a linear probe of 7.5 MHz. US examination was performed in 18 patients.
Computed tomography (CT) examination of the brain, abdomen, retro peritoneum, pelvis, and limbs was performed on the Siemens somatom DRH device. CT examination was performed in 23 patients. CT was performed in two series: a native and a series after i.v. application of non-ionic contrast medium (Omnipaque) 60 ml. The distance between the scans and the width of the scans is 10 mm, and the distance between the scans and the width of the scan for CT scan of the skeleton is 3 mm.
3. Results
Preoperative diagnosis was established by the clinical examination, serological tests (indirect hemagglutination test), X-ray examination, US and CT examination. Nonspecific abdominal pain and a nontender palpable abdominal mass were the most predominant symptoms, other symptoms varied according to the localization of the HC. Patients with brain HD presented with neurological symptoms. The diagnosis of HC was confirmed on histopathological examination of the specimen in all cases.
In our study, primary renal HD occurs in 7 patients. It can be asymptomatic, wherein most patients the clinical presentation includes lateral mass, renal colic, persistent fever, haematuria, dysuria, hydaturia, pyuria, renal calculi or hypertension, symptoms that are not specific to diagnosis.
The initial radiogram shows an increased contour of the affected kidney with a soft-tissue mass with scaly calcification. In the IVU dominates the suppression and obstruction of the duct system with subsequent hydronephrosis and parenchymal reduction, and the renal contour is deformed.
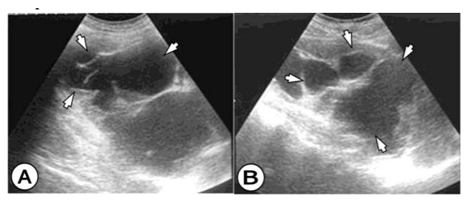
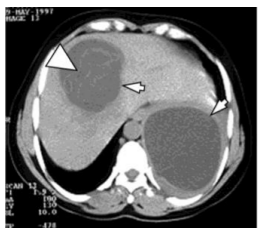
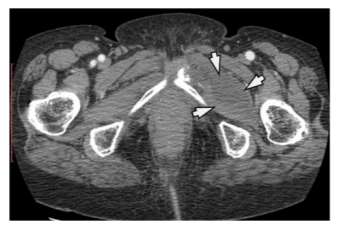
The US examination shows cystic tumor mass that deforms the kidney, suppresses the duct system, with subsequent hydronephrosis and parenchyma reduction. Nonfunctional kidney we have in 3 cases. (Figure 1) The HC is clearly limited, and in the interior, there are septas, daughters cyst, and peeled hydatid membranes.
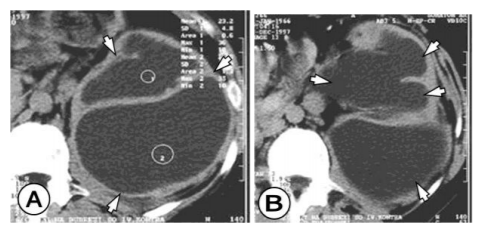
The CT finding consists of a unilocular or multilocular cyst, with a density of 8 to 27 HE, which most often involves both ends, with well-defined walls, which are with contrast enhancement. (Figure 2) Calcifications of the walls and daughters cystic which are with less density are often encountered in the cyst. (Figure 3) The presence of these two findings helps in establishing the right diagnosis.
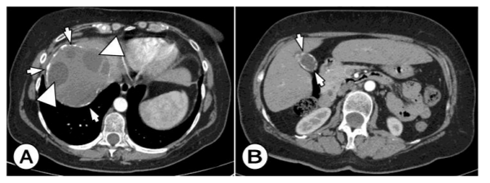
Primary involvement of the spleen from HD is very rare, we met in 3 cases. Secondary, it is involved in rupture of the HC of the liver or abdominal wall, with dissemination to the spleen. The clinical manifestation is nonspecific and consists of abdominal pain and an enlarged spleen.
US and CT findings consist of cystic spleen changes, fulfilled with fluid, hydatid sand, and cystic debris. (Figure 4) Wall calcifications, as well as multiple cysts, are often encountered in the large cyst.
Cerebral HD is also a rare and non-specific localization of the disease and is even more common in children than in adults in our material we encounter in 3 cases.
The clinical manifestation includes a headache, vomiting, edema of papillae or blurred vision. It is more often supratentorial, encompassing the area of a. cerebri media. The parietal lobe is the most common site of the development of the cerebral HC.
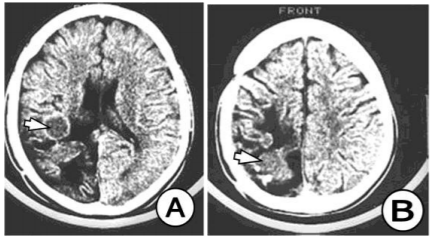
The CT image is typical and consists of a large, well-rounded or oval intraparenchymal lesion, which contains a fluid with a density approximate to the cerebrospinal fluid. (Figure Perifocal edema is not noticeable despite the sever mass effect and the existing hydrocephalus.)
In our study, the primary HD of the musculoskeletal system occurs in 3 patients, 2 on the skeleton and 1 in the soft tissues (muscles). The involvement of the skeleton of primary HD is rare and occurs in well-vascularized bone zones. Most commonly affected are vertebrae, epiphyses of the long bones, iliac bones, skull, ribs and soft tissues. In our material, in 1 cases the HC is on the spine, and in 1 cases in the long bones. The HC occupies only part of the vertebrae, and the remaining part of the cyst extends into the environment. After i.v. application of a contrast medium we have poor contrast enhancement of the pericyst. The lesions in the long bones are revealed as a consequence of a pathological fracture, and in the spine, the first sign is the compression of the cauda equina and spinal nerves.
CT image of bone lesions is similar to that seen on radiographs, a well-defined, multilocular, osteolytic lesion, with a honeycomb appearance, accompanied by an inflammation of the bone and a thinning of the cortex. CT accurately determines the boundary of destruction and clearly reveals spreading the process outside the bone in the surrounding soft tissues. There may be a typical picture of a cyst, round or oval, with sharp and thin edges, which poorly contrast enhancement, and the cyst content shows a liquid density of 8 - 32 HU. There is a clear border between the HC and the bone, and the bone in the surroundings of the cyst is of normal structure and morphology.
The HC in soft tissues manifests itself with a palpable mass that compresses the affected organs. US findings are used in the diagnosis of soft tissue HD, with cysts being shown in the shape of a grape or soap bubbles of different sizes. CT findings consist of unilocular or multilocular cysts, with an atypical solid component due to inflammatory changes, so that it can mimic the tumor. (Figure 6)
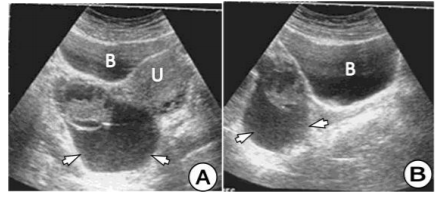
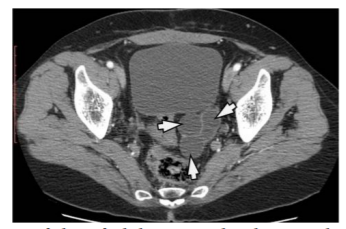
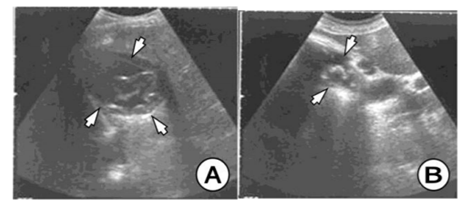
In a patient at age of 27, we found a primary multilocular HC in the left ovary. CT scan clearly demonstrates well-defined, multilocular HC in shape of soap bubbles of different sizes in the left ovary. (Figure 7) In another patient of age at the age of 19, we found an HC of ovarian, uterus and rectum, which on the US and CT had typical features for the HC. The patient was operated on and after three years of operation, a relapse of the HC on the right ovary was registered with US and CT. (Figure 8)
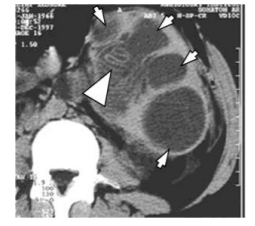
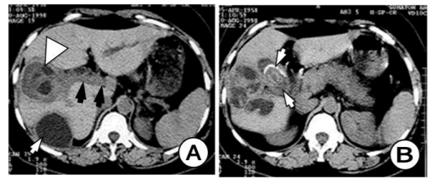
We have two cases with HC in the gallbladder, one with primary hydatidosis, and the second with a rupture of HC from the hepatic in the biliary ducts and gallbladder. (Figure 9) On US and CT there are two visible HC in the liver. The first one in the right lobe is inflamed with good delineation from the hepatic parenchyma with fibrous tissue, pericystic membrane or adventitia. (Figure 10) The same HC is penetrated into the biliary ducts with maintained fistula and communication with the biliary ducts as well as with gallbladder. The gallbladder is calculous with thicken and septated wall with present gallstones and filled by small hydatid daughter cysts. (Figure 11) The biliary ducts are widened especially the common hepatic duct in which the daughter cysts are seen as a cause for obstructive jaundice.
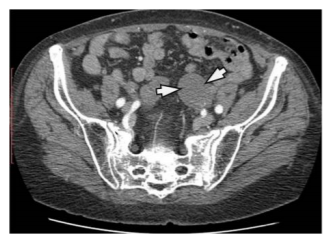
Peritoneal HD is also rare, in our material it appears in 3 patients, it can be located anywhere and is usually a consequence of the rupture of the hepatic, splenic or mesenteric HC. Cysts can cause abdominal distension or obstruction. On the SBFT and BE examinations of the digestive tube, it shows lateral pushing by the soft-tissue mass. Using US and CT, a clear cystic formation is obtained, clearly bounded by a thin wall, septated, with daughters cyst present. (Figure 12)
In a female patient at the age of 27, with the US examination of the breast, an HC was diagnosed with typical sonographic features, round multilocular cyst with multiple daughter cysts with a diameter of 45mm.
In all patients, surgery was performed and postoperative verification of the diagnosis set by US and CT was obtained.
4. Discussion
HD is endemic in the Middle East, Mediterranean countries, South America, North Africa, and Australia. [13]HD is an anthropozoonosis caused by Echinococcus species. The Echinococcus granulosus is the most common response for cystic formation, follow by Echinococcus multilocularis [4]. Humans represent intermediate hosts in parasite life cycle when occasionally ingest larvae. [1,2]The ingested larvae settle in the liver and other organs through the blood and lymph. The host reacts to the cyst by forming a fibrous capsule (pericyst), which contains blood vessels that provide nutrients for the parasite. From the germinal layer, scolices, brood capsules, and daughter cysts are formed by endoproliferation. [1,2]
Although HC disease can be found in almost all organs and tissues of the human body, it is most frequently seen in the liver (50–77%), the lungs (15–47%), the peritoneal cavity (8-12%), spleen (2-3%), the kidneys (1-4%), the brain (2%), the bones (1,5-2%), genitals (1%), muscles (0,5-1%)the spleen (0.5–8%), the kidneys (2–4%), and brain (up to 1%). [4,5,6,14,15]
Peritoneal hydatidosis is an uncommon finding. Only a few cases of primary peritoneal hydatidosis have been reported in the literature. [16,17] Secondary peritoneal hydatidosis is more common than primary. [18] Peritoneal HC, either primary or secondary, represents an uncommon but significant manifestation of this disease. [19] It is always secondary to traumatic or surgical rupture of a hepatic, spleen or mesenteric cyst. [18,20] CT is the modality of choice for these patients because it permits imaging of the entire abdomen and pelvis. The lesions are generally multiple and any type of HC can arise anywhere in the peritoneal cavity[18,21].
Clinically, nearly 30% of the patients with spleen HC are asymptomatic. Splenomegaly is the most frequent finding, which is incidentally determined. [9] The clinical symptoms caused by spleen HC mostly depend on the pressure effect of the cyst on the neighboring organs and the replacement of the neighboring organs[22].
US and CT scans, alone or in combination, can establish a definite diagnosis of spleen HC in almost all cases. The US is the primary preferred diagnostic method because it is inexpensive, easy, and has a high diagnostic value. It is diagnostic because it shows the cystic structure of the lesion, the presence of daughter vesicles, and hydatid sand. [11] CT is usually the next step after a US diagnosis has been made. The main purpose is to visualize the relationship between the HC and the surrounding tissue. [11] Although CT scan is more sensitive than the abdominal US, non-calcified benign cysts without daughter cysts cannot be differentiated per se from other benign cysts either by CT or by the US.[11,22-24] Differential diagnostic include epidermoid cyst, pseudocyst, solitary abscess, hematoma and cystic neoplasm of the lien. The diagnosis is easy when the HD has a multifocal localization and involves multiple organs and when daughter cysts are present[21,23,24].
Although hydatid cyst disease can be present in all parts of the body, isolated renal involvement is a rare condition, comprising only 2-4% of all cases. [25] The kidney is the most common location in the urogenital system. Renal HC causes symptoms with vague pain or a palpable mass in the lumbar region.[26,27] In our case, the HC was identified incidentally. In our case, the lesion was located in the right kidney. Imaging methods are important for early diagnosis. Although the US is the most useful radiologic method, unenhanced CT has a high sensitivity for lesions. [12,25,26,28,29] The cyst can be unilocular or multilocular septal in case of active cysts. Renal hydatid cysts usually remain symptomless for years[26,29]
Isolated renal hydatid cyst disease may reside symptomless for a long time. Clinical presentations include fever, malaise, flank pain, palpable mass, hematuria, and hydaturia. [30,31] Although hydaturia which is characterized by the presence in urine of grape skin–like fragments, is the only pathognomonic sign that testifies of a ruptured hydatid cyst in the urinary tract, it has been seen only in 5%-25% of renal hydatid cyst disease.[30,31] In our case, the patient had dull pain and palpable mass in the right flank with loss of weight and urinary symptoms such as nocturia and incontinence. However, there was no evidence of hydaturia. In the world literature, HD of the bladder is extremely rare, and in our practice so far we have not registered such a case.
Intracranial HD is rare, with a reported incidence of 1-2% of all cases with HD. [32] Cerebral HD is more common in the pediatric population. [33] Intracranial hydatid can occur anywhere within the brain but are more frequently located in the supratentorial compartment, especially in the middle cerebral artery territory. [32] The parietal lobe is the most common site with 69%, the other less common sites are a skull, cavernous sinus, eyeball, pons, extradural, cerebellum, and ventricles[32,34,35].
The cerebral HC are benign and slowly growing cysts and present late when they increase in size and become large. A headache and vomiting were the most commonly reported symptoms in other series as well as in our patients. Other symptoms such as hemiparesis, seizures, visual field alteration and gait disorders, may vary with the location of the cyst. [35] Cerebral HC is commonly solitary. Multiple cerebral HC are rare[34,36].
Intracranial HC may also be classified as primary or secondary. In primary multiple cysts, each cyst has a separate pericyst with brood capsule scolices and these originate from multiple larvae affecting brain after crossing the gastrointestinal tract, liver, lungs and right side of the heart without affecting them.[34] The secondary multiple cysts result from spontaneous, traumatic or surgical rupture of the primary intracranial HC and they lack brood capsule and scolices. [34] CT scans characteristically show HC as a spherical, well defined, an enhancing cystic lesion without peripheral edema. [10] The fluid density is generally equal to that of CSF. A fine rim of peripheral enhancement with perilesional edema may be seen in the presence of active inflammation. [10] In contrast to hydatid cysts, arachnoid cysts are not spherical in shape and not surrounded entirely by brain substance. Arachnoid cysts are extra-axial masses that may deform the adjacent brain. Cystic tumors of the brain could be differentiated by the enhancement of the mural nodule. When a pyogenic abscess shows a cyst like central necrotic area, peripheral edema is almost always present and the rim enhances intensely following contrast administration. [10,32] The treatment of hydatid cyst is surgical and the aim of surgery is to excise the cyst in toto without rupture to prevent recurrence and anaphylactic reaction.
In the differential diagnosis of HC of the brain arachnoid cyst, porencephalic cyst, cystic tumor and abscess should be excluded. The arachnoid cyst is not round and is not surrounded by brain tissue like HC. The porencephalic cyst usually interacts with the ventricular system. The cystic tumor usually has a soft-tissue component, which is enhancement after i.v. application of contrast medium. The abscess, after i.v. administration of contrast medium shows the walls enhancement and has a surrounding edema[32].
The involvement of the female genital tract in hydatid disease is extremely rare but the most important factor in its diagnosis is the awareness of the possibility of hydatid disease especially in the endemic area. [37,38] Ovarian involvement is also very rare and generally secondary to the peritoneal spread of daughter cysts due to rupture of a liver HC. [38,39] Ovarian HC is usually asymptomatic and they can be discovered incidentally or they may cause irritation or compression symptoms. [37-39] The ovarian lesion may be unilocular or contain daughter cysts that can give rise to a multiloculated appearance, and ovarian lesion should be considered in the differential diagnosis of cystic pelvic masses such as cystadenoma or cystadenocarcinoma [37-39].
Radiological diagnosis of osseous HD is also difficult because of non-specific findings Radiography usually reveals single or multiple osteolytic lesions and in some cases shows cortical thinning, osteosclerosis, and pathologi-cal fractures. [40,41] Intraosseous foci of HD predominate in spongious bone tissues and consist of small, separate, and thin-walled cysts. While these cysts expand, they destroy the surrounding trabeculae and can reach a considerable size. [40,42,43] HC can mimic an image similar to an abscess or tumor, which is difficult to distinguish from a malignant bone tumor. Signs that mean much for a differential diagnosis include the osteoporosis zone, bone thickening of the affected bone, and calcification in the lesion. The association of the bone lesion with calcification in the surrounding soft tissue allows a reliable diagnosis of the HC [40,42-45].
Ultrasound and CT scan of abdomen and pelvis may help to determine the exact location and nature of a cystic pelvic mass, its relationship with adjoining structures, vascular invasion, operability and also exclude hydatid disease elsewhere[44,45]
Spinal HC can be manifested by different clinical features. It is considered to be relatively silent, slowly progressive disease with a latent period of many years. [40,46] Early symptoms appear when the small cyst expands and produces significant mechanical compression on surrounding vital structures. At this stage, intermittent pain can be present as a symptom. As the disease progresses and the cyst enlarges, the patient starts suffering from a persistent backache, gradually leading to the severe neurologic deficit and varying degrees of weakness of limbs. When HD affects the spine, tuberculous spondylitis appears as the most common differential diagnostic problem. The absence of destruction on the discal surfaces of the vertebrae and the spread of the disease through subperiosteal and subligamentous pathways are typical of vertebral HD. [46,47]
Muscle HD is rare, possibly because of muscle lactic acid content and muscle contraction, two factors that likely prevent cyst growth in striated muscle. Clinically, the early stage of muscular HD is asymptomatic. The symptoms and signs of HC disease depend on the involved organ, site of localization, effect on the adjacent tissue, complications after rupture. A palpable mass is the most constant clinical finding of HD affecting soft tissues, and the clinical manifestations are caused by compression of the organ involved. [49,50] Muscle HD most often manifests as a slow-growing soft-tissue tumor and can imitate myositis or a calcified hematoma.
A common complication of the hydatid cyst of the liver is its penetration into the biliary ducts. The US, and especially CT study gives enough information for successful diagnosis and further successful surgery.[51]CT examination enables excellent space presentation of pathologic process, which is especially important for an operator for surgery planning approach, techniques, and procedures which will be applied for improving the pathological process. Primary hydatid cyst of the gallbladder is an extremely rare entity. [52-54] There are reports of the gallbladder daughter cysts secondary to liver cysts[55,56].
Gold standard treatment is the total excision of the cyst, avoiding its rupture and spillage; anaphylactic shock is a major risk connected. Surgical treatments include complete cyst excision in most of the patients. Nephrectomy, cholecystectomy, ovarian cystectomy, distal pancreatectomy, and splenectomy, were performed whenever cysts invaded these organs.
Indications for surgery include large cysts with multiple daughter cysts, superficial location amenable to rupture, cysts exerting pressure on adjacent organs, and cysts in ectopic locations such as seminal vesicle, brain, bones, spleen, kidneys.
5. Conclusion
Echinococcosis can appear at any site of the human body, therefore in endemic areas, whenever we have cystic formations or unidentified tumor masses in the differential diagnosis, HD should also be considered.
US imaging has great value in the screening of HD, it is cheap, easily accessible, and simple to perform a method, with high diagnostic accuracy. CT provides precise topographic and morphological features of the pathological substrate, with high sensitivity, specificity, and accuracy.
We can conclude that imaging methods have a great diagnostic value in HD with atypical localization, in its verification and preoperative evaluation.
References
- McManus D. P, W. Zhang, J. Li, and P. B. Bartley. Echinococcosis. The Lancet. 2003, 362, 9392, 1295–1304.
- Eckert J, Deplazes P. Biological, epidemiological, and clinical aspect of Echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004; 17(1):107-35.
- Dakkak A. Echinococcosis/hydatidosis: a severe threat in Mediterranean countries. Vet Parasitol 2010; 174: 2-11.
- Polat P, Kantarci M, Alper F, Suma S, Koruyucu MB, Okur A. Hydatid cyst from head to toe. Radiographics. 2003; 23:475–94.
- Pedrosa I, Saiz A, Arrazola J, Ferreiros J, Pedrosa CS. Hydatid disease: radiologic and pathologic features and complications. Ra-diographics 2000; 20: 795-817.
- Yuksel et al. Hydatid Disease Involving Some Rare Locations in the Body: a Pictorial Essay. Korean J Radiol 2007;8:531-540
- Delis SG, Bakoyiannis A, Exintabelones T, Triantopoulou C, Papailiou J, Dervenis C. Rare localizations of the hydatid disease.Experience from a single center. J Gastrointest Surg. 2007; 11:195–8.
- Akkaya H, Akkaya B, Gonulcu S. Hydatid disease involving some rare sites in the body. Turkiye Parazitol Derg 2015; 39: 78-82.
- Kiresi DA, Karabacakoglu A, Odev K, Karakose S. Uncommon locations of hydatid cysts. Acta Radiol. 2003; 44:622–36.
- Yasar B, Serdar K, Hasan N, Umit O, Adnan C, Masum S. Cerebral hydatid disease: CT and MR imaging findings. Swiss Med Wkly. 2004; 134: 459–467
- Von-Sinner WN, Stridebeck H. Hydatid disease of the spleen. Ultrasonography, CT and MR imaging. Acta Radiol 1992; 33: 459–61.
- Rexiati M, Mutalifu A, Azhati B, Wang W, Yang H, Sheyhedin I, et al. Diagnosis and surgical treatment of renal hydatid disease: a retrospective analysis of 30 cases. PLoS One 2014; 9: e96602.
- Hunter GW, Strickland GT. Hunter’s Tropical medicine and emerging infectious diseases. 8th ed. Philadelphia: Saunders; 2000. pp. 866–76.
- Tsaroucha AK, Polychonidis AC, Lyrantzopoulos N.Hydatid disease of the abdomen and other locations. World J Surg. 2005; 2013:1161-5.
- Tuzun M, Hekimoglu B. Various locations of cystic and alveolar HD: CT appearances. J Comput Assist Tomogr 2001; 25:81–87.
- Kumar KS. A Case of Primary Peritoneal Hydatidosis. Med J Armed Forces India. 2009; 65:278–279.
- Hegde N, Hiremath B. Primary peritoneal hydatidosis. BMJ Case Rep. 2013;2013
- Majbar MA, Souadka A, Sabbah F, Raiss M, Hrora A, Ahallat M. Peritoneal echinococcosis: anatomoclinical features and surgical treatment. World J Surg. 2012; 36:1030–5.
- Singh S, Reddy M. A rare case of primary peritoneal hydatid cyst. Int J Reprod Contracept Obstet Gynecol. 2017 Jan;6(1):360-363
- Wani I, Lone AM, Hussain I, Malik A, Thoker M, Wani KA. Peritoneal hydatidosis in a young girl. Ghana Med J. 2010; 44:163–4.
- Varama A, Mahajan A, Sandhu GS, Singh M, Prasad N. Intraperitoneal, pelvic and retroperitoneal multiple hydatid cyst. Arch Int Sur 2014;4:186-9
- Durgun V, Kapan S, Kapan M, Karabiçak I, Aydogan F. Primary splenic hydatidosis. Dig Surg 2003; 20: 38–41.
- Temiz A, Albayrak Y, Er S, Albayrak A, Aslan OB. Primary splenic hydatidosis: Case series. Arch Clin Exp Med. 2017; 2(2):31-34.
- Rasheed K, Zargar SA, Telwani AA. Hydatid cyst of spleen: a diagnostic challenge. N Am J Med Sci 2013; 5: 10–20.
- Kumar SA, Shetty A, Vijaya C, Geethamani V. Isolated primary renal echinococcosis: a rare entity. Int Urol Nephrol 2013; 45: 613-6.
- Angulo JC, Sanchez-Chapado M, Diego A, Escribano J, Tamayo JC, Martin L. Renal echinococcosis: clinical study of 34 cases. The Journal of urology. 1997; 157:787-94.
- Hota D, Pujari S, Choudhuri S, Panda S. Isolated Renal Hydatidosis Presenting as Renal Mass: A Diagnostic Dilemma. Urol Case Rep. 2015; 3:103-5.
- Razzaghi MR, Mazloomfard MM, Bahrami-Motlagh H, Javanmard B. Isolated renal hydatid cyst: diagnosis and management. Urol J. 2012; 9:718-20.
- Goğuş C, Safak M, Baltaci S, Turkolmez K. Isolated renal hydatidosis: experience with 20 cases. J Urol 2003; 169: 186-9.
- Mongha R, Narayan S, Kundu AK. Primary hydatid cyst of kidney and ureter with gross hydatiduria: A case report and evaluation of radiological features. Indian J Urol 2008; 24: 116-7.
- Madani AH, Enshaei A, Pourreza F, Esmaeili S, Madani MH. Macroscopic Hydatiduria: An Uncommon Pathognomonic Presentation of Renal Hydatid Disease. Iranian journal of public health. 2015;44:1283
- Abbassioun K, Amirjamshidi A. Diagnosis and management of hydatid cyst of the central nervous system: Part 2 : hydatid cysts of the skull, orbit and spine. Neurosurgery, Vol. 11, March 2001: 10–16.
- Erashin Y, Mutluer S, Guzelbag E : Intracranial hydatid cysts in children. Neurosurgery 1993; 33: 219-224.
- Alzain TJ, Alwitry SH, Khalili HM. Multiple intracranial hydatidosis. Acta Neurochirurgica. 2002, 144, 1179–1185.
- Nur A, Murad B, Hakan H, Bülent E. Central nervous system hydatidosis in Turkey: a cooperative study and literature analysis of 458 cases. J. Neurosurgery. 2000, July, Vol.93: 1-8.
- Radmenesh F, Nejat F. Primary Cerebral Hydatid Cyst: Two Cases Report. Iran J Pediatr. Mar 2008;Vol 18 (No 1); 83-86
- Fatnassi R, Turki E, Majdoub W, Hammami S, Hajji M Primary Ovarian Hydatid Cyst: A Case Report and Review of Literature. Insights Reprod Med. (2017) Vol.1 No.1:5
- Cattorin L, Trastulli S, Milani D, Cirocchi R, Giovannelli G, Avenia N, Sciannameo F. Ovarian hydatid cyst: A case report. International Journal of Surgery Case Reports 2 (2011) 100–102
- Adewunmi O.A., Basilingappa H.M. Primary ovarian hydatid disease in the Kingdom of Saudi Arabia. Saudi Med J. 2004;25(November (11):1697–1700.
- Bracanovic et al.: Skeletal manifestations of hydatid disease in Serbia: Demographic Distribution, Site Involvement, Radiological Findings, and Complications. Korean J Parasitol Vol. 51, No. 4: 453-459, August 2013
- Booz MY. The value of plain film findings in hydatid disease of bone. Ciln Radiol 1993; 47: 265-268
- Papanikolaou A. Osseous hydatid disease. Trans R Soc Trop Med Hyg 2008; 102: 233-238.
- Torricelli P, Martinelli C, Biagini R, Cristofaro R. Rdiographic and computed tomographic finding in hydatid disease of bone. Skeletal Radiol (1990)19: 435-439
- Kotoulas G, Gouliamos A, Kalovidouris A, Vlahos L, Papavasiliou C. Computed tomographic localization of pelvic hydatid disease. Eur J Radiol (1990)11: 38-41
- Bhatnagar N, Kishan H, Sura S, Lingaiah P, Jaikumar K. Pelvic Hydatid Disease: A Case Report and Review of Literature. Journal of Orthopaedic Case Reports 2017 Jul-Aug: 7(4):Page 25-28
- Prabhakar MM, Acharya AJ, Modi DR, Jadav B. Spinal hydatid disease: a case series. J Spinal Cord Med 2005; 28: 426-31.
- Herrera A, Martinez AA, Rodriguez J. Spinal hydatidosis. Spine 2005; 30: 2439-2444.
- Gopal N, Chauhan S, Yogesh N. Primary spinal extradural hydatid cyst causing spinal cord compression. Indian J Orthop 2007; 41: 76-78.
- Martin J, Marco V, Zidan A, Marco C. Hydatid disease of the soft tissues of the lower limb: findings in three cases. Sceletal Radiol (1993) 22: 511-514
- Tekin R et al. - Hydatid cyst in muscles: clinical manifestations, diagnosis, and management of this atypical presentation. Rev Soc Bras Med Trop 48(5):594-598, Sep-Oct, 2015
- Vakhidov AV, Il'khamov FA, Strusskii LP, Azat'ian TS. Diagnosis and treatment of liver echinococcosis complicated by cysto-biliary fistula. Khirurgiia-Mosk 1998; 15-7.
- Krasniqi A, Limani D, Gashi-Luci L, Spahija G, Dreshaj IA. Primary hydatid cyst of the gallbladder: a case report. J Med Case Rep. 2010; 4:29.
- Kabiraj P, Kuiri SS, De U. Primary hydatid cyst of gallbladder: A case report. Int J Case Rep Images 2015; 6(7):440–443.
- Safioleas M, Stamoulis I, Theoqaris S, Moulakakis K, Makris S, Kostakis A: Primary hydatid disease of the gallbladder: a rare clinical entity. J Hepatopancreat Surg 2004, 11:352-6.
- Raza MH, Harris SH, Khan R: Hydatid cyst of gallbladder. Indian J Gastroenterol 2003, 22:67-68.
- Ivanis N, Rubinic M, Gudovic A, Zeider F: Ultrasound image of an echinococcus daughter cyst in the gallbladder. Ultraschall Med 1994, 15:269-71.