Information
Journal Policies
Stewart-Treves Syndrome in a Patient with Breast Cancer on FDG-PET/CT Imaging
Pelin Ozcan Kara1*, Zehra Pinar Koc1, Ozgur Turkmenoglu2, Kadir Eser3, Emel Sezer3
2.Mersin University, Faculty of Medicine, Department of General Surgery, Mersin, Turkey.
3.Mersin University, Faculty of Medicine, Department of Oncology, Mersin, Turkey.
Copyright : © 2017 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Lymphangiosarcoma is a rare malignant tumor which occurs as a complication of chronic lymphedema. 18-F-Flourodeoxyglucose (FDG) Positron Emission Tomography/Computed Tomography (PET/CT) is usually used in a variety of cancer patients for staging, re-staging and treatment response. We present a rare case of lymphangiosarcoma with a history of right mastectomy, axillary lymphadenectomy and chemoradiotherapy 14 years ago in a 73-year-old woman who presented with a mass on her right lymphedematous arm. FDG-PET/CT demonstrated the second primary tumor and its subcutaneous spread.
Keywords: Stewart-Treves syndrome, Angiosarcoma, Lymphedema, Breast Cancer.
1. Introduction
Angiosarcoma is a rare entity in breast cancer patients. 18-F-Flourodeoxyglucose (FDG) Positron Emission Tomography/ Computed Tomography (PET/CT) is usually used in breast cancer patients for staging, re-staging and treatment response. Lymphangiosarcoma also called as Stewart-Treves Syndrome can be develop in the following years as a complic-ation after therapy of breast cancer. Imaging and staging are of special importance at this stage. FDG PET-CT imaging can be useful in these patients. In this case report, the role of FDG PET/CT in the demonstration of angiosarcoma and its distribution in a patient with metastatic breast cancer is presented.
2. Case Report
A 73-year-old woman who presented with a mass on her right lymphedematous arm was sent to our department for FDG-PET/CT imaging for restaging. She had a history of mastectomy and axillary lymphadenectomy and also chemo radiotherapy for right breast cancer 14 years ago. Her arm had become persistently swollen in the recent weeks and a clinical diagnosis of lymphedema was made, initially, until she developed an ulcerated mass lesion. Biopsy of the lesion was angiosarcoma.
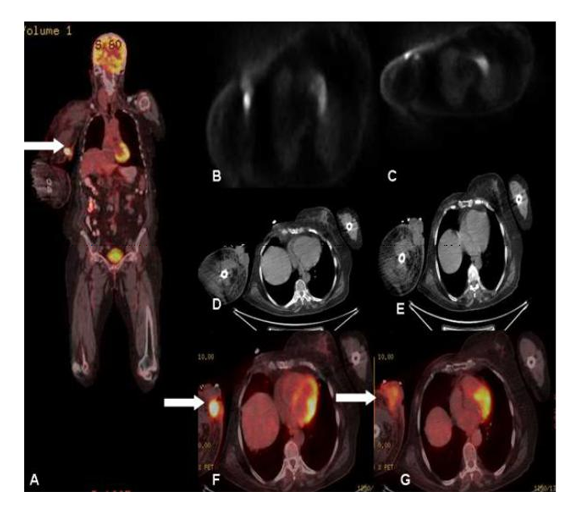
Immunohistochemical studies revealed positi-vity for CD34, CD31, and a high Ki-67 proliferation rate (70%). FDG-PET/CT scan was performed for restaging. Following 12 h of fasting, while blood glucose level was 102 mg/dl, 8.77 mCi 18 F-FDG i.v. was injected. After 60 minutes, the patient was imaged in the 3D mode 2.3 minutes per bed from the calvarium to the footwell. Obtained images were evaluated after attenuation correction with low dose nondiagnostic CT. Right breast was not seen secondary to operation. No pathologic focus was found in the left breast, left and right axilla and right breast operation area on PET-CT scan. Lymphedema was seen in the right arm. PET-CT scan demonstrated an approximately axial 57x48 mm heteroge- nous lobulated mass extending to skin and subcutaneous tissue and with borders seper- ated from bone and muscle structures in the right arm with a SUVmax of 17.05 (Figure 1). In the vicinity of the mass, satellite nodules with the largest size of about 2.5 cm were observed. Fibrotic changes were observed in right apical lung region secondary to radiotherapy. No additional pathologic focus was detected on whole body imaging. Accor-ding to PET-CT findings amputation of right arm was performed.
3. Discussion
Lymphangiosarcoma is a very rare vascular neoplasm with poor prognosis occur as a complication of chronic congenital or acquired lymphedema. Lymphangiosarcoma arising in lymphedematous tissue have been reported in the literature in a variety of reports. As seen in this case, it is most frequently associated with postmastectomy lymphedema also called as Stewart-Treves syndrome [1]. Stewart-Tre ves syndrome is an angiosarcoma that occurs because of chronic lymphedema. In most cases, lymphangiosarcoma is a complication after mastectomy with axillary node dissection and postoperative radiation. Patients usually have a significant history for breast cancer and radical mastectomy, 5–15 years before sarcoma presentation. The mean onset between radical mastectomy and lymphangiosarcoma is 11 years [2]. Stewart-Treves syndrome accounts for approximately 5% of angiosarcomas [3]. Lymphangiosarcoma can exceptionally arise in congenital hereditary lymphedema also called as Milroy syndrome and Meige syndrome and nonhereditary lymphedema such as congenital, praecox, or forme tarde lymphedemas [4-6]. In the majority of cases in literature, purplish papules or nodules, necrosis or ulceration have occurred in upper extremities, 10 to 20 years after radical mastectomy, and radiotherapy [7-11]. L Cui et. al reported 10 cases in a case report and review of literature [12]. In a recent case report and review of the literature [13] the authors reported that there are approximately 400 cases of Stewart-Treves syndrome reported in the literature, most in postmastectomy patients who are predominantly women. PET-CT imaging is a valuable method in restaging of breast cancer, especially in distant metastases. Although, reports describing FDG PET-CT findings of lymphangiosarcoma are very rare [14-18]. As a complication after therapy of breast cancer, lymphangiosarcoma can be develop in following years and imaging is also of special importance at this stage. The most common site of metastasis are the lungs, followed by liver, bone, soft-tissue structures and lymph nodes in lymphangiosarcoma if untreated [2, 19-21]. For the demonstration of subcutaneous spread and planning of a surgical procedure, F-fluorodeoxyglucose positron emission tomography may be used [2, 22]. In this case report, the role of FDG PET-CT in the demonstration of angiosarcoma and its subcutaneous spread in a patient with known metastatic breast cancer is presented. FDG PET-CT demonstrated the second primary tumor and its distribution. There was no distant metastasis in this case. High SUVmax values were obtained in proportion to aggressivity of the second malignancy. One of the satellite nodules was observed at the level of the right anterior humerus head in PET-CT images. Additional wide resection could not be performed for local control and right arm amputation was performed instead of wide resection to the patient according to PET-CT findings. PET-CT was found useful in this case especially for subcutaneous spread of the disease and excluding distant metastasis.
4. Conclusion
Stewart-Treves syndrome is a rare complic-ation of postmastectomy lymphedema with very poor prognosis. Patient’s survival can be improved by early diagnosis and knowing the disease distribution in the diagnosis. FDG PET-CT imaging as a whole body imaging procedure can be used for demonstrating both subcutaneous spread and distant metastasis. There are only few case reports on this entity. More reports on PET-CT imaging in lymphan-giosarcoma patients are needed.
References
- Stewart FW, Treves N. Lymphangiosarcoma in postmastectomy lymphedema: a report of six cases in elephantiasis chirurgica. Cancer. 1948;1:64–81.
- Sharma A, Schwartz RA: Stewart-Treves syndrome: pathogenesis and management. J Am Acad Dermatol 2012;67:1342–1348.
- McHaffe DR, Kozak KR, Warner TF, Cho CS, Heiner JP, Attia S: Stewart-Treves syndrome of the lower extremity. J Clin Oncol 2010;28:351– 352.
- Kettle EH. Tumours arising from endothelium Proc R Soc Med. 1918;11:19 –34.
- Bostrom LA, Nilsonne V, Kronberg M, et al. Lymphangiosarcoma in chronic hereditary oedema (Milroy’s disease). Ann Chir Gynecol Fenniae. 1989;78:320–323.
- Goodman RM. Familial lymphedema of the Meige’s type. Am J Med. 1962;32:651– 656.
- Mackenzie DH. Lymphangiosarcoma arising in chronic congenital and idiopathic lymphoe-dema. J Clin Path. 1971;24:5244 –5529.
- Merrick TA, Erlandson RA, Hajdu SI. Lymp-hangiosarcoma of a congenitally lymphae-dematous arm. Arch Path. 1971;10:365–371.
- Dubin HV, Creehan PE, Headington JT. Lymphangiosarcoma and congenital lymphe- dema of the extremity. Arch Dermatol.1974;110:608–614.
- Sordillo PP, Chapman R, Hajdu SI, et al. Lymphangiosarcoma. Cancer. 1981;48:1674 –1679.
- Tasdemir A., Karaman H, Unal D., Mutlu H. Stewart-Treves Syndrome after Bilateral Mastectomy and Radiotherapy for Breast Carcinoma: Case Report. J Breast Health 2015;11(2);92-94.
- Cui L, Zhang J, Zhang X, Chang H, Qu C,Zhang J, Zhong D. Angiosarcoma (Stewart-Treves syndrome) in postmastectomy patients:report of 10 cases and review of literature. IntJ Clin Exp Pathol. 2015 Sep 1;8(9):11108-15.
- Berebichez-Fridman R, Deutsch YE, Joyal TM, Olvera PM, Benedetto PW, Rosenberg AE, Kett DH. Stewart-Treves Syndrome: A Case Report and Review of the Literature. Case Rep Oncol. 2016 Apr 1;9(1):205-11.
- Kara PO, Gedik GK, Sari O, Kara T, Yilmaz F. FDG-PET/CT in a Patient With Lymph-angiosarcoma. Clinical Nuclear Medicine Volume 35, Number 6, 428-429, 2010.
- Sharma P., Singh H, Singhal A, Bal C, Kumar R. Detection of Recurrent Cutaneous Angiosarcoma of Lower Extremity with 18F-Fluorodeoxyglucose Positron Emission Tomo graphy-Computed Tomography: Report of Three Cases. Indian J Dermatol. 2013 May-Jun; 58(3): 242.
- Roy Gottlieb, Rukhsana Serang, David Chi, Harry Menco. Stewart-Treves syndrome. Radiol Case Rep. 2012; 7(4): 693
- Jung Ho Lee, Yeon Jin Jeong, Deuk Young Oh, Sang Wha Kim, Jong Won Rhie, Sang Tae Ahn. Clinical Experience of Stewart-Treves Syndrome in the Lower Leg. Arch Plast Surg. 2013 May; 40(3): 275–277.
- Chen YR, Hsieh TC, Yen KY, Kao CH. Distant metastases in a young woman with Stewart- Treves syndromedemonstrated by an FDG- PET/CT scan.Clin Nucl Med. 2014 Nov;39(11):975-6.
- Young RJ, Brown JN, Reed MW, Hughes D, Woll PJ: Angiosarcoma. Lancet Oncol 2010;11:983–991.
- Yamane H, Ochi N, Tabayashi T, Takigawa N: Stewart-Treves syndrome after cervical cancer.Int Med 2012;51:513.
- Grobmyer S, Daly J, Glotzbach R, Grobmyer A: Role of surgery in the management of postmastectomy extremity angiosarcoma (Stewart-Treves syndrome). J Surg Oncol 2000;73:182–188.
- di Meo N, Drabeni M, Gatti A, Trevisan G: Letter: A Stewart-Treves syndrome of the lower limb. Dermatol Online J 2014;18:1–2.