Information
Journal Policies
Colonization by Group B Streptococcus and Antimicrobial Resistance Patterns in Kuwait: An Observational Study in 2014 and 2016
Fatemah Al-Mutairi1,2, Mohammad Sedeqi3, Retaj Mohammad4, Ellen Stobberingh5, Jan Hendrik Richardus6*
2.Department of Microbiology Farwaniya Hospital, P.O Box 13373 Farwaniya 81004 Kuwait
3.Department of Microbiology Farwaniya Hospital, P.O Box 13373 Farwaniya 81004 Kuwait
4.University College Dublin, Belfield Dublin 4, Ireland
5.Department of Infectious Diseases, Municipal Public Health Service. Schiedamsedijk 95, 3011 EN Rotterdam, The Netherlands
6.Department of Public Health, Erasmus MC, University Medical Center Rotterdam, P.O. Box 2040, 3000 CA Rotterdam, The Netherlands
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: We determined the colonization rate of Group B streptococcus (GBS) and the antibiotic resistance profiles among pregnant women in Kuwait, and established the association between the colonization rate and age group and clinical status.
Methods: The study was in Farwaniya Hospital in Kuwait in 2014 and 2016. Included were pregnant women ≥ 35 weeks of gestation, of whom vaginal specimens were tested. Data were also collected on neonatal infections, including swabs from eyes, ears and skin. Basic descriptive statistical analysis was applied.
Results: We included 2,830 women and 1,525 neonates. The GBS colonization rate in women was approximately 20% in both years. There was a (statistically not significant) higher colonization rate of 20.0% among women aged 26-30 years compared to the lowest colonization rate of 18.5% in the > 30 years’ age group in 2014 (p = 0.763); a similar (statistically significant) trend is shown in 2016 (p = 0.010). In all women with GBS, 6% were positive for vaginitis and 25% for Candida. There was no statistical significant association between GBS colonization and vaginitis in 2014 (p = 0.965) and 2016 (p = 0.091), but there was an association between GBS colonization and candidiasis in both years. Overall, 25.3% and 24.1% of pregnant women carrying GBS were infected with Candida in 2014 and 2016, respectively (p > 0.05). All GBS isolates were susceptible for penicillin G, cefotaxime and vancomycin, while approximately 20% were resistant to clindamycin and erythromycin. GBS infection in neonates was around 4%.
Conclusion: GBS colonization rates in women and infection in neonates where comparable in 2014 and 2016. GBS colonization rates were higher in women of non-Kuwaiti origin, coming mainly from India, Pakistan, Philippines, and Bangladesh.
Group B Streptococcus, pregnant women, neonates, antibiotic resistance,Public Health and Community Medicine
1. Introduction
Group B streptococcus (GBS), also identified as Streptococcus agalactiae, are part of the vaginal microbiota. GBS are non-motile, non-spore forming, catalase negative Gram-positive cocci, which grow facultative anaerobically. On blood agar, GBS appear as smooth, flat, grayish color colonies with a diameter of 0.6-1.2μm [1]. GBS can cause meningitis, sepsis, and pneumonia, especially in newborn infants. In (female) adults GBS can be the causative agent of urinary tract infections and postpartum infections [2].
Infants with GBS infections usually present with a range of symptoms such as respiratory distress and sepsis within the first 24-48 hours of life [2]. GBS is a leading cause of death among newborn infants globally. The type and severity of infections depend on the age of the newborn. Infections occurring within the first week of life are classified as early onset disease whereas infections occurring in the later neonatal period are called late onset infections [3]. Risk factors for early onset GBS disease include low birth weight, preterm birth and premature rupture of membranes. The incidence of neonatal infections due to GBS ranges between 0.5 and 4% whereas the incidence of GBS colonization in pregnancy ranges between 5 and 30% [4].
Penicillin G is the preferred and most common drug used to treat GBS infections. However, 12% of pregnant women are reported to be allergic to penicillin and therefore alternative options such as erythromycin and clindamycin are suggested [3]. In case of allergy to both types drugs, vancomycin, teicoplanin or cephalosporin are recommended [1]. Because GBS is acknowledged as a primary cause of neonatal infections, it is vital to control the disease. According to the American College of Obstetricians and Gynaecologists, antenatal screening of pregnant women is highly recommended to control and manage GBS infections. This screening approach involves all pregnant women between the 35th and 37th week of pregnancy [1] and includes the collection of vaginal swabs for culture. Patients of which the swabs are positive for GBS are treated with antimicrobial therapy [4].
The objectives of this study were i) to determine the prevalence of GBS and antibiotic resistance profiles among pregnant women in Kuwait, and ii) to establish the association between the colonization rate, age group and clinical status (vaginitis and candidiasis).
2. Methods
This study was based on data from the Farwaniya Microbiology Laboratory of the Farwaniya Hospital, a public hospital of 1,200 beds in Kuwait serving one million patients annually. The hospital has an obstetrics and gynecology department that delivers 8,000 children annually.
The laboratory logbook was reviewed by the first author for the periods January 1st to June 30th in 2014 and 2016, respectively. All pregnant women ≥ 35 weeks of gestation who attended the antenatal care clinic during those two periods and from whom vaginal specimens were tested were included in this study. In the same study period, another data set was collected regarding neonatal infections, including swabs from eyes, ears and skin (umbilicus) collected at the labor room for the presence of GBS.
A screening policy was introduced in 2015. All pregnant women ≥ 35 weeks of gestation were asked to provide a vaginal swab between 35 and 37 weeks of gestation for examination for GBS. If positive, a notification was sent to the labor ward prior to labor so that the patient received antibiotic therapy at least 4 hours before labor or during labor. In addition, the pediatric ward was also notified to test the baby directly after birth and provide treatment if necessary (if any bacterial infection was present). Prior to 2015, no screening policy was in place and therefore the treatment for infected newborn babies was delayed.
The vaginal swabs were collected according to the current Center of Disease Control (CDC) guidelines from women ≥ 35 weeks of gestation. Swabs from the low vagina were collected and placed into a non-nutritive transport medium (Stuart’s media) [2]. The swabs were processed in the Farwaniya microbiological laboratory according to standard methods [1]. The swabs were inoculated onto Tryptic soy agar plate with a concentration of 5% sheep blood (BA), chocolate agar plate and Sabouraud agar plate. The chocolate agar plates were incubated at 5% CO2 for 48 hours at 37°C and the other plates at 37°C for 48 hours. From all colonies growing on Sabouraud agar a wetmount preparation was done and if yeast cells were visible the germ tube tests were performed. This was done by inoculating the colonies in undiluted plasma followed by incubation for 4 hours at 37°C and microscopically assessed for the presence of germtubes.
From each neonate three swabs were collected (eye, ear and skin) and inoculated on blood agar and MacConkey plate under aerobic conditions at 37°C for 48 hours. At the same time, the swabs were further cultured on chocolate agar plate and optochin disk was added and incubated under CO2 at 30°C for 48 hours. Finally, the ear swabs were cultured on Sabouraud’s agar plates, which were incubated at 30°C and read after 48 hours.
The vaginal swabs were Gram stained and examined for bacterial vaginosis using the Nugent criteria, and the presence of clue cells and Candida. The smears were then evaluated for Candida vaginitis and were assessed for a possible correlation with GBS colonization [5].
After incubation of the agar plates, beta-hemolytic colonies were Gram stained and the Gram-positive cocci showing negative results with catalase testing were directly examined with Strept B grouping latex reagent. All the remaining negative cultures were left for further incubation for another 24 hours and then re-examined.
The GBS strain BAA-611 was used as a control for susceptibility testing. Five different antibiotic disks were used: Penicillin G (10 M), clindamycin (2 M), erythromycin (50 M), vancomycin (30 M) and cefotaxime (30 M).
Clinical & Laboratory Standards Institute (CLSI) 2014 was used as a reference method (https://clsi.org/). The disk diffusion results were translated using the standard interpretative measures suggested by the National Committee of Clinical Laboratory Standards.
GBS colonization was established for 2014 and 2016 and described as frequencies and percentages. This was also done for the risk factors associated with GBS colonization. Antibiotic susceptibility profiles (susceptible vs. resistant) were described as percentages of the total number of isolates tested. For the evaluation of the efficiency of the screening policy, the number and percentage of positive individuals was determined and then compared between the two time periods.
3. Results
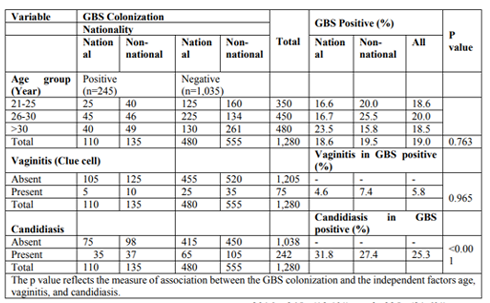
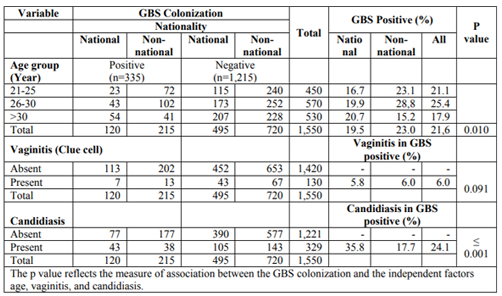
In the two observation periods, 1,280 (2014) and 1,550 (2016) women were included in the study. Of these women, 46% (590/1,280) and 40% (615/1,550) were Kuwaiti nationals in, respectively. Of the women screened for GBS colonization during the antenatal care program in 2014 and 2016, 245 (19.1%) and 335 (21.6%) were confirmed positive for GBS. There was a higher colonization rate of 20.0% among women belonging to the 26-30-year age group compared to the lowest colonization rate of 18.5% in the > 30 age group in 2014 (p = 0.763). A similar and now statistically significant trend is shown in 2016 (p = 0.010). In 2014, there was a high colonization rate of 19.5% among non-nationals compared to 18.6% among nationals (Table 1). In 2016, the higher colonization rate of 23.0% was found among non-nationals compared to 19.5% among Kuwaiti nationals (Table 2). There was no statistical significant association between GBS colonization and vaginitis in 2014 (p = 0.965) and 2016 (p = 0.091). According to the Gram stain results, there was an association between GBS colonization and candidiasis in both years. Among the Kuwaiti women who were colonized with GBS in 2014, 31.8% also had Candida, compared to 27.4% in non-Kuwaiti women (p < 0.001). In 2016, the results were 35.8% and 17.7% (p ≤ 0.001), respectively. Overall, 25.3% and 24.1% of pregnant women carrying GBS were infected with Candida in 2014 and 2016, respectively (p > 0.05).
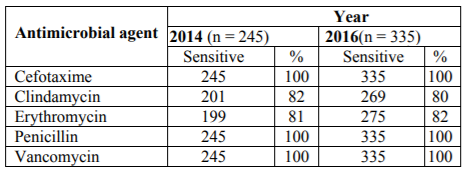
The antibiotic susceptibility was determined for 245 and 335 GBS isolated in 2014 and 2016, respectively (Table 3). All isolates were susceptible for penicillin G, cefotaxime and vancomycin. Forty-four (18%) and 46 (19%) isolates were resistant to clindamycin and erythromycin in 2014, and 66 (20%), 60 (18%) in 2016.
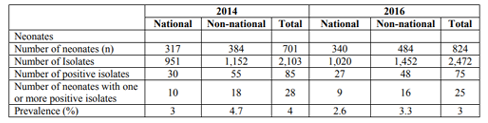
A total of 2,103 and 2,472 swab samples from eye, ear, and skin were obtained from 701 and 824 neonates in 2014 and 2016, respectively. Of these, 45% (951/2103) and 41% (1020/2472) were from Kuwaiti nationals in 2014 and 2016, respectively. In total 35% (30/85) and 36% (27/75) samples of Kuwaiti nationals were positive for GBS in 2014 and 2016, respectively. The prevalence of GBS infection in neonates was 4% and 3% in 2014 and 2016, respectively (Table 4).
4. Discussion
We found a slight, statistically not significant, increase in the rate of GBS colonization among pregnant women, from 19.0% in 2014 to 21.6% in 2016. Over half of the women in the study were of non-Kuwaiti origin. In 2014 and 2016, the GBS colonization rates were higher in these women than in those of Kuwaiti origin, in particular in the age group 26-30 years. Non-Kuwaiti women come from many countries, the most important being India, Pakistan, Philippines, Bangladesh, Egypt, Syria, Iran, Lebanon and Jordan.
Our results are comparable to studies in both developed and developing countries [6]. In our study, GBS colonization in 2016 was 21.6%, which was higher than in previous studies in Kuwait in 2002 and 2004; with rates of 14.2% and 16.4%, respectively [4,7]. These studies used different methodology and study populations. The rate appears to increase over time, which could be due to the relative increase in the non-Kuwaiti (migrant) population. The population of Kuwait remains rising with a growth rate in 2016 of 4.79%, mainly attributed to the growth of the migrant population [8].
GBS colonization rates differ per country. For example, low GBS rates ranging from 10-17% were found in Iran, United Arab Emirates, Tunisia and Hong Kong, whereas high rates of 23-32% were reported in Egypt, Tanzania and Saudi Arabia [9]. These differences might be caused by ethnic or geographic factors. It has been established that the GBS colonization rate is independent of the socioeconomic status of a country [10].
We examined antibiotic susceptibility profiles and compared these over the 2-year time period. All isolates were susceptible to cefotaxime, vancomycin and penicillin G. Resistance to clindamycin was 18% and 20% in 2014 and 2016, respectively, and to erythromycin 19% and 18%, respectively. A study in Kuwait in 2011 reported that GBS resistance to clindamycin was 7% and erythromycin 12.6% [6]. This study however, included a different patient population in a different hospital and used a different methodology. This increase in resistance is possibly explained by an increase of these antibiotics globally, and in Kuwait in particular. Erythromycin resistance to GBS is increasing globally; for instance it was 20% in Korea and 53% in Brazil [11, 12]. Penicillin remains the first drug of choice for intra-partum antibiotic prophylaxis for GBS colonization among pregnant women. Erythromycin is the drug of choice for women with penicillin allergies who are colonized with GBS [2] and depending on the type of allergy also a cephalosporin can be used as alternative.
Regarding the relationship between GBS colonization and age group, we found the highest rate of GBS colonization (20.0%) and (25.4%) in the 26-30-year age group in 2014 and 2016, respectively, followed by the 21-25-year age group; 18.6% and 21.1%, respectively. The age group with the lowest GBS rate was the >30-year group; 18.5% and 17.9% in 2014 and 2016, respectively. In 2014, age group and GBS colonization rate were not associated (p = 0.763). However, there was a statistically significant difference between age group and GBS colonization in 2016 (p = 0.010). This might be due to the greater number of subjects included in 2016 (1,550) in comparison to 2014 (1,280). Our findings were in contrast to the results of Al-Sweih et al. in 2003, who described no significant difference in age group and GBS colonization (p = 0.890) [4].This could be explained by differences in methodology and study population i.e. individuals from different cultural backgrounds’ were evaluated.
Only 5.8% and 6.0% of the pregnant women with GBS also had vaginitis in 2014 and 2016, respectively. These findings argue against a clear correlation between GBS colonization and vaginitis. This might be due to the limited diagnostic method to diagnose vaginitis (i.e. only Gram stains were used). In a previous study in 2004, 5.2% of the GBS carriers had also vaginitis, thus, indicating a stable rate of vaginitis in Kuwait[4]. We did find an association between GBS colonization and candidiasis, as 25.3% of GBS carriers reported candidiasis in 2014 and 24.1% in 2016. The percentages in both years were higher in Kuwaiti women compared to non-Kuwaiti women. In Brazil, a similar association was found [13]. On the other hand, a study by Hoing et al. showed no association between GBS colonization and candidiasis [14].
This study has strengths and weaknesses. A strength is the large sample size of 1,280 and 1,550 women in 2014 and 2016, respectively. In addition, this study included a control group of women ≥35 weeks of gestation during two comparable time periods in 2014 and 2016. A weakness is the use of a single vaginal swab and the lack of use of an enrichment broth, whereas the CDC recommends 2 swabs (rectal and vaginal) to study GBS colonization and a selective enrichment broth containing chromogenic substrates. Finally, no external risk factors such as smoking or the prevalence of sexually transmitted diseases were investigated in this study.
5. Conclusion
We conclude that GBS colonization rates in women and infection in neonates where comparable in 2014 and 2016, and that GBS colonization rates were higher in women of non-Kuwaiti origin, coming mainly from India, Pakistan, Philippines, and Bangladesh.
This study was based on anonymized information from the hospital and laboratory database. Ethics approval was given by the Standing Committee for Coordination of Medical Research of the State of Kuwait Ministry of Health on its meeting (#2/2016) held on Tuesday February 23, 2016.
FAM designed the study, collected and analyzed the data and wrote the manuscript. MS and RM helped collecting and analyzing the data. ES provided microbiological expertise to the design and analysis of the study, and advised during the writing of the paper. JHR contributed to the design of the study and supervised the analysis and writing of the manuscript. All authors read and approved the final manuscript.
References
- Ghaddar N, Alfouzan W, Anastasiadis E, Al Jiser T, Itani SE, Dernaika R, Eid T, Ghaddar A, Charafeddine A, Dhar R, El Hajj H. Evaluation of chromogenic medium and direct latex agglutination test for detection of group B streptococcus in vaginal specimens from pregnant women in Lebanon and Kuwait. J Med Microbiol. 2014;63(Pt 10):1395-9.
- Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases NCfI, Respiratory Diseases CfDC, Prevention. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1-36.
- Capanna F, Emonet SP, Cherkaoui A, Irion O, Schrenzel J, Martinez de Tejada B. Antibiotic resistance patterns among group B Streptococcus isolates: implications for antibiotic prophylaxis for early-onset neonatal sepsis. Swiss Med Wkly. 2013;143:w13778.
- Al-Sweih N, Maiyegun S, Diejomaoh M, Rotimi V, Khodakhast F, Hassan N, George S, Baig S. Streptococcus agalactiae (Group B Streptococci) carriage in late pregnancy in Kuwait. Med Princ Pract. 2004;13(1):10-4.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2) :297-301.
- Boswihi SS, Udo EE, Al-Sweih N. Serotypes and antibiotic resistance in Group B streptococcus isolated from patients at the Maternity Hospital, Kuwait. J Med Microbiol. 2012;61(Pt 1):126-31.
- Hammoud MS, Thalib L, Maiyegun SO. The epidemiology of group B streptococcal colonization among obstetrical and newborn populations in Kuwait. Int J Gynaecol Obstet. 2002;76(3):315-6.
- Anonymous. Kuwait Population 2017 2018 [Available from: http://worldpopulationreview. com/ countries/kuwait-population/.
- Khan MA, Faiz A, Ashshi AM. Maternal colonization of group B streptococcus: prevalence, associated factors and antimicrobial resistance. Ann Saudi Med. 2015;35(6):423-7.
- Capan M, Mombo-Ngoma G, Akerey-Diop D, Basra A, Wurbel H, Lendamba W, Auer-Hackenberg L, Mackanga R, Melser J, Belard S, Ramharter M. Epidemiology and management of group B streptococcal colonization during pregnancy in Africa. Wien Klin Wochenschr. 2012;124 Suppl 3:14-6.
- Uh Y, Jang IH, Hwang GY, Yoon KJ, Song W. Emerging erythromycin resistance among group B streptococci in Korea. Eur J Clin Microbiol Infect Dis. 2001;20(1):52-4.
- Betriu C, Culebras E, Gomez M, Rodriguez-Avial I, Sanchez BA, Agreda MC, Picazo JJ. Erythromycin and clindamycin resistance and telithromycin susceptibility in Streptococcus agalactiae. Antimicrob Agents Chemother. 2003;47(3):1112-4.
- Rocchetti TT, Marconi C, Rall VL, Borges VT, Corrente JE, da Silva MG. Group B streptococci colonization in pregnant women: risk factors and evaluation of the vaginal flora. Arch Gynecol Obstet. 2011;283(4):717-21.
- Honig E, Mouton JW, van der Meijden WI. The epidemiology of vaginal colonisation with group B streptococci in a sexually transmitted disease clinic. Eur J Obstet Gynecol Reprod Biol. 2002;105(2):177-80.