Information
Journal Policies
Clinical Problems of Congenital and Perinatal Cytomegalovirus (CMV) Infection
Masayuki Nagasawa, M.D., Ph.D
Copyright :© 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Congenital CMV (cytomegalovirus) infection occurs in one out of 1,000 births and remains a serious health problem for children both in the developed and developing countries. It has been shown that perinatal CMV infection also has a risk for impaired psychomotor development. Although the strategy for congenital CMV infection is an early detection and treatment, effective prevention or treatment methods remain to be developed so far in spite of remarkable medical progress. It is important to disseminate accurate clinical information widely and promote further investigations.
congenital CMV infection, sensorineural hearing loss, ganciclovir, hyperimmune globulin, dried blood sample, Pediatrics
CMV (cytomegalovirus) is a virus of the herpes genus existing universally in the world and every human being is infected once during one’s life. It may present sometimes with fever and hepatitis when infected, but it is almost self-limiting or asymptomatic and not serious in nature. However, when CMV infects the neonates, and immunocompromised hosts, it will be serious and fatal eventually. CMV can infect various cells such as mesenchymal cells (fibroblasts, vascular endothelial cells, smooth muscle cells), epithelial cells, hematopoietic cells, and neuronal cells[1]. Once, infected primarily, it continues to infect latently in the myeloid progenitor cells as an episomal form, and is reactivated and induces various complications in the patients under immunosuppressive conditions[2]. Molecular mechanism of CMV reactivation is being disclosed and it is considered that TNF-α and cyclic AMP are important triggering factors[3]. CMV infection is also known to induce thrombosis or microangiopathy[4], for which precise mechanisms are not well understood[5]. In the field of transplantation medicine, the control of CMV infection is one of the most important clinical concerns[6].
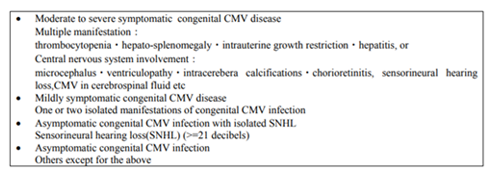
Meanwhile, CMV infects the fetuses via the placenta through a viremia in the primarily infected pregnant mothers, and induces congenital CMV infection, resulting in the serious sequels such as intrauterine growth retardation, hydrocephalus, mental retardation, sensorineural hearing loss, thrombocytopenia, and so on (Table-1)[7]. Recently, the number of uninfected pregnant mothers is increasing through the improvement of the hygiene environment, and there is a growing concern for the increased risk of congenital CMV infection. It is estimated that symptomatic congenital CMV infection occurs in one out of 1,000 births. However, it is considered that there are three to four folds asymptomatic congenital CMV patients, who are diagnosed later as a sensorineural hearing loss of unknown etiology[8]. Until now, a majority of a congenital sensorineural hearing loss other than genetic diseases is considered to be caused by asymptomatic congenital CMV infection[9].
The protective efficacy of the high titer anti-CMV antibody containing immunoglobulin (hyperimmune globulin) administration is still controversial. The effect of hyperimmune globulin to prevent congenital CMV infection has been reported in several non-randomized or retrospective clinical studies[10-13]. However, recent randomized clinical study in USA could not show significant preventive effect [14].
The fact that effective vaccine for CMV has not been developed so far is another serious problem. Up to date, preliminary data has been published that vaccine of monomeric recombinant CMV envelope glycoprotein B (gB) with MF59 adjuvant may prevent non-infected human mother from primary CMV infection during pregnancy, and reduce congenital CMV infection by 43%.[15] It is known that the natural conformation of human CMV gB within the viral envelope is a trimer, and the development of trimer gB has been investigated. Immunization of mice with trimeric human CMV gB induced up to 11-fold higher serum titers of total gB-specific IgG relative to monomeric human CMV gB.[16] It is also recognized that congenital CMV infection could develop through CMV reactivation of the pregnant mother already infected and immunized, although infectivity is much lower than in case of primary infection.9 Furthermore, CMV is secreted in breast milk, and infects neonates after birth, especially premature newborns[17].Recent report indicates that there is still a risk for impaired psychomotor development even in the case of primary infection in the early stage of a newborn period[18].
At present, the strategy for congenital CMV infection is an early detection and treatment of CMV infection, so far[19]. Effective antiviral drug available right now includes intravenous ganciclovir and foscarnet in Japan. For congenital CMV infection, the long-term anti-viral treatment for 6 months has been reported to reduce the severity or progression of hearing and psychomotor impairment[20,21]. The possible side effects such as myelosuppression and renal impairment are problems to be discussed. However, it has been reported that most of the neonates are well tolerable to a long-term ganciclovir administration. To monitor during treatment, it is recommended that absolute neutrophil counts should be followed weekly for 6 weeks, then at 8th week, and monthly thereafter[22]. For the former, oral medicine; valganciclovir is developed and is reported as effective as ganciclovir and beneficial for the long-term treatmentofneonates or early infants practically[21].
This new oral medicine may solve the serious blood access problem frequently experienced in neonates with low birth weight. In the recent consensus recommendation, valganciclovir is only recommended for neonates with moderate to severe symptomatic congenital CMV infection because of its possible adverse effects, cost-benefit and limited clinical effectiveness. Also, preventive use is not recommended for the same reasons above[22].
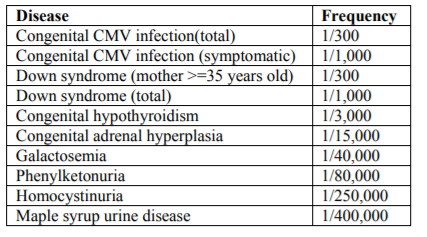
The highly sensitive diagnostic method of CMV infection is a polymerase chain reaction (PCR). It is shown that dried blood sample (DBS) used in mass screening for newborns is available as a specimen to detect CMV by PCR[23]. Recently, DBS is also used for screening of primary immunodeficiency[24]. The frequency of congenital CMV infection is higher than the other diseases which are targeted for present mass screening system as presented in Table-2.
Several clinical studies which evaluated the efficacy of universal CMV infection screening by PCR with DBS have been reported. Most of the reports have concluded that sensitivity of PCR to detect CMV in DBS is not sufficient enough as a screening method for congenital CMV infection clinically[25-28] Detection of CMV DNA in the saliva or urine by PCR is a most sensitive method at present and it is now considered that DBS should be used for confirmation of congenital CMV infection but not for universal screening[22,28] Recently, the result of hearing-targeted congenital CMV infection screening program has been published from Utah in USA[29]. In this program, infants who failed newborn hearing screening test were placed for CMV detection program as early as possible. In spite of remarkable medical progress, congenital CMV infection remains largely unrecognized in the developed and developing world[30]. It is now a major infectious cause of sensorineural hearing loss and psychomotor developmental defects in infants born in developed countries,[31] and second only to cerebral palsy in all causes of serious malformation in many countries. It is important to disseminate accurate clinical information and insidious thread of congenital CMV infection widely and promote further clinical investigation and basic scientific research of CMV to conquer this problem.
References
- Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Current topics in microbiology and immunology 2008; 325: 63-83.
- Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. The Journal of general virology 2006; 87(Pt 7): 1763-79.
- Stein J, Volk HD, Liebenthal C, Kruger DH, Prosch S. Tumour necrosis factor alpha stimulates the activity of the human cytomegalovirus major immediate early enhancer/promoter in immature monocytic cells. The Journal of general virology 1993; 74 ( Pt 11): 2333-8.
- Mitsuiki N, Tamanuki K, Sei K, Ito J, Kishi A, Kobayashi K et al. Severe neonatal CMV infection complicated with thrombotic microangiopathy successfully treated with ganciclovir. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy 2017; 23(2): 107-110.
- Justo D, Finn T, Atzmony L, Guy N, Steinvil A. Thrombosis associated with acute cytomegalovirus infection: a meta-analysis. European journal of internal medicine 2011; 22(2): 195-9.
- Koval CE. Prevention and Treatment of Cytomegalovirus Infections in Solid Organ Transplant Recipients. Infectious disease clinics of North America 2018; 32(3): 581-597.
- Coll O, Benoist G, Ville Y, Weisman LE, Botet F, Anceschi MM et al. Guidelines on CMV congenital infection. Journal of perinatal medicine 2009; 37(5): 433-45.
- Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. The New England journal of medicine 2001; 344(18): 1366-71.
- Schleiss MR, Permar SR, Plotkin SA. Progress Toward Development of a Vaccine Against Congenital Cytomegalovirus Infection. Clinical and vaccine immunology : CVI 2017.
- Nigro G, Adler SP, La Torre R, Best AM. Passive immunization during pregnancy for congenital cytomegalovirus infection. The New England journal of medicine 2005; 353(13): 1350-62.
- Nigro G, Adler SP, Parruti G, Anceschi MM, Coclite E, Pezone I et al. Immunoglobulin therapy of fetal cytomegalovirus infection occurring in the first half of pregnancy--a case-control study of the outcome in children. The Journal of infectious diseases 2012; 205(2): 215-27.
- Buxmann H, Stackelberg OM, Schlosser RL, Enders G, Gonser M, Meyer-Wittkopf M et al. Use of cytomegalovirus hyperimmunoglobulin for prevention of congenital cytomegalovirus disease: a retrospective analysis. Journal of perinatal medicine 2012; 40(4): 439-46.
- Visentin S, Manara R, Milanese L, Da Roit A, Forner G, Salviato E et al. Early primary cytomegalovirus infection in pregnancy: maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2012; 55(4): 497-503.
- Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. The New England journal of medicine 2014; 370(14):1316-26.
- Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML et al. Vaccine prevention of maternal cytomegalovirus infection. The New England journal of medicine 2009; 360(12): 1191-9.
- Cui X, Cao Z, Wang S, Lee RB, Wang X, Murata H et al. Novel trimeric human cytomegalovirus glycoprotein B elicits a high-titer neutralizing antibody response. Vaccine 2018; 36(37): 5580-5590.
- Maschmann J, Hamprecht K, Dietz K, Jahn G, Speer CP. Cytomegalovirus infection of extremely low-birth weight infants via breast milk. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2001; 33(12): 1998-2003.
- Brecht KF, Goelz R, Bevot A, Krageloh-Mann I, Wilke M, Lidzba K. Postnatal human cytomegalovirus infection in preterm infants has long-term neuropsychological sequelae. The Journal of pediatrics 2015; 166(4): 834-9 e1.
- Pass RF. Congenital cytomegalovirus infection and hearing loss. Herpes : the journal of the IHMF 2005; 12(2): 50-5.
- Kimberlin DW, Lin CY, Sanchez PJ, Demmler GJ, Dankner W, Shelton M et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. The Journal of pediatrics 2003; 143(1): 16-25.
- Kimberlin DW, Jester PM, Sanchez PJ, Ahmed A, Arav-Boger R, Michaels MG et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. The New England journal of medicine 2015; 372(10): 933-43.
- Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. The Lancet. Infectious diseases 2017; 17(6): e177-e188.
- Scanga L, Chaing S, Powell C, Aylsworth AS, Harrell LJ, Henshaw NG et al. Diagnosis of human congenital cytomegalovirus infection by amplification of viral DNA from dried blood spots on perinatal cards. The Journal of molecular diagnostics : JMD 2006; 8(2): 240-5.
- Borte S, von Dobeln U, Fasth A, Wang N, Janzi M, Winiarski J et al. Neonatal screening for severe primary immunodeficiency diseases using high-throughput triplex real-time PCR. Blood 2012; 119(11): 2552-5.
- Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW, Jr., Palmer AL et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. Jama 2010; 303(14): 1375-82.
- Meyer L, Sharon B, Huang TC, Meyer AC, Gravel KE, Schimmenti LA et al. Analysis of archived newborn dried blood spots (DBS) identifies congenital cytomegalovirus as a major cause of unexplained pediatric sensorineural hearing loss. American journal of otolaryngology 2017; 38(5): 565-570.
- Yamaguchi A, Oh-Ishi T, Arai T, Sakata H, Adachi N, Asanuma S et al. Screening for seemingly healthy newborns with congenital cytomegalovirus infection by quantitative real-time polymerase chain reaction using newborn urine: an observational study. BMJ open 2017; 7(1): e013810.
- Wang L, Xu X, Zhang H, Qian J, Zhu J. Dried blood spots PCR assays to screen congenital cytomegalovirus infection: a meta-analysis. Virology journal 2015; 12: 60.
- Diener ML, Zick CD, McVicar SB, Boettger J, Park AH. Outcomes From a Hearing-Targeted Cytomegalovirus Screening Program. Pediatrics 2017; 139(2).
- Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, de Lima Isaac M, de Carvalho e Oliveira PF, Boppana S et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2009; 49(4): 522-8.
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Reviews in medical virology 2007; 17(4): 253-76.