Information
Journal Policies
Headache in Children and Adolescents
B.D.Gupta,Mukesh Kumar
2.Consultant Pediatrician, Shriram Hospital, Jodhpur, Rajasthan, India.
Copyright :© 2017 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Headaches are among the most common neurological disorders in children and adolescents. Headache disorders represent a major public health problem and have been underestimated, under-recognized and under-treated throughout the world. Primary headaches, especially migraine and tension-type headaches are the most important cause of headaches in children and adolescents. Long term trends showing a significant increase in incidence and prevalence of headache over the years, reflecting untoward changes in children’s lifestyle. Medication overuse headache is also becoming a worldwide health problem with increasing prevalence. Epidemiological studies have established a strong association between primary headaches with psychiatric, neurological, cardiovascular and other medical disorders. Treatment of headache requires correct and timely diagnosis followed by a comprehensive treatment program and is often a challenging task in children and adolescents. Newer drugs that target the inflammatory mediators and receptors involved in the pathophysiology of headaches are under development. More long-term comprehensive population-based studies and research studies needed to understand the pathophysiology and mechanism as well as in the development of drugs for management of childhood headaches.
Headache, Migraine, Tension-type headache, Children, Adolescents
Headaches are among the most common neurological disorders in children and defined as pain located above the orbito-meatal line (cephalgia) not limited to the distribution of a cranial or cervical nerve (neuralgia). Primary headaches (especially migraine and tension-type headache (TTH)) are the most important cause of headaches in children and adolescent. It has detrimental effects on the child‟s academic performance, cognitive profile, and interpersonal relationships; and associated with personal and societal burdens of pain, disability,damaged quality of life and immense economic losses [1-5].
2. Classification
The International Headache Society (IHS) classifies headache into primary and secondary headache disorders, and set diagnostic criteria in the International Classification of Headache Disorders 3rd edition - beta version (ICHD III beta) for primary headaches, based on clinical history and physical examination. Primary headaches cannot be attributed to another underlying disorder, and are most often recurrent and episodic in their presentation. Migraines and tension type headaches are the most common primary headaches in children [2,7].
A migraine is characterized by episodic attacks, moderate to severe in intensity, focal in location on the head, having a throbbing quality, and may be associated with nausea, vomiting, photophobia and phonophobia. Compared to the adults, pediatric migraine is shorter in duration and has a bilateral, often bi-frontal location. A migraine can also be associated with an aura that may be typical (visual, sensory, or dysphasic) or atypical (i.e., hemiplegic, “Alice in Wonderland” syndrome). While, TTH is mild to moderate in severity, diffuse in location, not affected by activity, non-throbbing and often described as constant pressure. TTH is much less frequently associated with nausea, photophobia or phonophobia, and is never associated with more than one of these at a time or with vomiting [2].
When the headaches become frequent, they convert into chronic daily headaches (CDH) in up to 1% of children. The risk of conversion into a daily headache becomes more prominent when a headache occurs more than 15 days a month [2].
Secondary headache disorders result when another underlying organic disorder leads tocephalgia. The underlying illness should be clearly present as a direct cause of the headaches. Secondary headaches may be associated with significant morbidity and therefore warrant more urgent evaluation, require additional tests to confirm or exclude certain etiologies and perhaps even empirical treatment [2,7].
3. Epidemiology
The prevalence of childhood headaches and migraines has been reported from across the world with widely variable estimates of prevalence. Majority of the information available is limited to hospital-based studies or school surveys which may not reflect the true dimensions of the disorders prevalent in a community. Among the most common representative primary headaches in children, some studies point migraines and others as tension type headache [8-11].
Headache prevalence ranges 3-8% at 3 years,19.5% at 5years, 37-51.5% at 7 years, and26-82%in 7–15year-olds [12-17].In a review of 50 population-based studies, the overall prevalence of a headache and migraine in children and adolescents (< 20years) at any point in time, was 58.4% and 7.7%, respectively. This analysis showed that around 60% of children and adolescent are prone to headaches and 8% prone to migraines, over periods varying from 3 months to lifetime [18].In another review, covering 64 cross-sectional epidemiological studies published in 32 different countries in past 25 years, the mean prevalence of headaches and migraines in children and adolescents was 54.4% and 9.1%, respectively [19].The prevalence of a tension-type headache in children in population and school-based surveys estimated about 31% (10%–72%) [6].The prevalence revealed an upward trend with increasing age for overall headache causes, migraine and TTH [17,20,21]. There was a preponderance for female sex for overall headache causes (67% in females versus 58% in males) and migraine (9.7% in females versus 6% in males) [18,20-23].
Long term trends show significant increase in incidence and prevalence of migraine and headache over the last 30 years, more so in developing countries, probably reflecting untoward changes in children‟s lifestyle.
Incidence rates of migraine with aura and migraine without aura increased from 5.2 and 14.5 per 1000 person-years in 1974 to 41.3 and 91.9 per 1000 person-years in 2002, respectively [24].
There was a statistically significant difference in the prevalence of migraine between Europe (8.35%) and the Middle East (8.69%) on the one hand and the USA (6.58%) and the Far East (6.70%) on the other, probably due to combination of genetic predisposition as well as environmental factors .
A positive family history of headaches in first degree relatives was elicited in 23-66.18% of migraineurs and in 35-40% of TTH patients [25-27]. In another study, 46% of patients with a migraine were found to have a family history of migraines as compared to 18% of patients with migraine having a family history of TTH [28]. The study showed that the risk and frequency of TTH were found to be higher in patients with a migraine [29].Stress that included mental stress, sleep deprivation, physical exertion, watching television for long hours, etc. were the most common trigger factors for all primary headache disorders in general and more so for a migraine (85.06%) than TTH (43.08%)[30]. Menstruation as a trigger factor was reported in up to 1/3rd of the cases .
Secondary headaches are uncommon in patients with recurrent headaches. Non-life-threatening diseases, in particular, respiratory tract infections and minor head trauma, are the most common causes of secondary headaches. In a small minority of patients, headache is secondary to serious life-threatening intracranial disorders, most common being meningitis [31].
In one study, 20% of the study population had a headache once or more times a week, with an average Pediatric Migraine Disability Assessment Scale score (PedMIDAS) of over 12.1 (and an impact on over 12 days in a 3-month period). 10% of the population had a PedMIDAS score of 16.8 and a generic Pediatric Quality of Life Inventory (PedsQL4) score of 70.1, indicating a poorer quality of life than that of children with asthma, diabetes, or cancer [32]. The primary headache disorders contributed to 34.61% of the total school days lost in a calendar year . An average of 0.6 days of school was lost in a 3-month period across all school children [32].
Global Burden of Disease Study 2010shows that TTH (20.1%) and migraine (14.7%) are respectively the second and third most prevalent disease globally. Migraine was globally 8th and 4th in south Asia region in rank in leading causes of years lived with disability (YLDs). There was 8.0% and 8.1% rise in all age YLDs per 100000 from Migraine and TTH, from 1990 to 2010, respectively [33]. WHO, International Headache Society, World Headache Alliance and European Headache Federation, in 2004, jointly launched a global campaign against headache disorder-“Lifting the Burden", to raise awareness of headache disorders and improve the quality of headache care and access to it worldwide.
Being so prevalent worldwide, still, only a minority of people with headache disorders are diagnosed appropriately and timely. Even in developed countries, one-quarter of the parents were not aware of their children's headache [34]. Among children ultimately diagnosed with a primary headache syndrome, 21% are previously misdiagnosed by a healthcare professional, including neurologists. The time to correct diagnosis is 1 year on average and increases to 3 years if a child has been previously misdiagnosed [35,36]. The average migraine headache history in one study was 2.48 +/- 1.18 years in girls and 2.57 +/- 1.18 years in boys[37]. Over 50% of children and adolescent are not visiting a health care provider about their headache and less than 20% taking prevention [38]. Even in developed country like United States, one fourth of migraineurs are candidates for preventive therapy, and most of them are not receiving it, indicating an unmet need for it [39]. Adolescents with headache often prefer taking analgesics themselves and overuse it, than visiting a physician. Medication overuse headache (MOH) had become a worldwide health problem with a prevalence of 1-2%, and is refractory to most of the available treatment options [40].
4. Pathophysiology
Brain parenchyma itself is pain insensitive. Headache pain arise from pain-sensitive structures which include intracranial arteries and venous sinuses, dura matter and cranial nerves and from the extra cranial structures such as the skin, muscles, and blood vessels in the head and neck; mucosa of the sinuses and dental structures [41]. Migraine is a multi-factorial, neurovascular headache disorder with complex pathophysiology, which has not been fully clarified. Migraine attacks are likely to begin centrally, suggest that multiple neuronal systems function abnormally and involve multiple cortical, subcortical, and brainstem areas that regulate autonomic, affective, cognitive, and sensory functions. There is a genetic predisposition for generalized neuronal hyper excitability, extreme sensitivity to fluctuations in homeostasis and a decreased ability to habituate itself, leading to the recurrence of a headache.
Symptoms in the prodromal phase point to the potential involvement of the hypothalamus, brainstem, cortex, and limbic system. Hypothalamic neurons respond to changes in physiological and emotional homeostasis and regulate the preganglionic parasympathetic neurons in the superior salivatory nucleus, which in turn, stimulate the release of acetylcholine, vasoactive intestinal peptide, and nitric oxide (NO) from meningeal terminals of postganglionic parasympathetic neurons in the sphenopalatine ganglion, leading to dilation of intracranial blood vessels, plasma protein extravasation, and release of inflammatory molecules capable of activating pial and dural branches of meningeal nociceptors.
Migraine aura is caused by cortical spreading depression (CSD), a slowly propagating wave of depolarization followed by hyperpolarization in cortical neurons and glia. CSD releases calcitonin gene-related peptide (CGRP) and NO into the meninges, which leads to transient constriction and dilatation of pial arteries and the development of dural plasma protein extravasation, neurogenic inflammation, platelet aggregation, and mast cell degranulation, which introduces proinflammatory molecules, altering the molecular environment of meningeal nociceptors.
Headache phase begins with consequential activation of meningeal nociceptors at the origin of the trigeminovascular system leading to sensitization and activation of peripheral trigeminovascular neurons.
The trigeminovascular pathway conveys nociceptive information from the meninges and large cerebral arteries, through the thalamus to the higher cortical structures of brain- brainstem nuclei, hypothalamic nuclei, and basal ganglia nuclei, which construct the specific nature of migraine pain. These projections are involved in initiating nausea, vomiting, yawning, lacrimation, urination, loss of appetite, fatigue, anxiety, irritability, and depression by the headache itself. When central trigeminovascular neurons become sensitized, their spontaneous activity increases, their receptive fields expand, and they begin to respond to innocuous mechanical and thermal stimulation of cephalic and extra cephalic skin areas as if it were noxious.Recent evidence suggests that hypothalamic and brainstem neurons lower the threshold for the transmission of nociceptive trigeminovascular signals from the thalamus to the cortex.
Genetic predisposition in migraine is evidenced by family history and was first observed in patients with familial hemiplegic migraine (FHM). Three genes identified, which regulates transmitter release, glial ability to clear (reuptake) glutamate from the synapse, and the generation of action potentials. FHM1 encodes the pore-forming α1 subunit of the P/Q type calcium channel on chromosome 19; FHM2 encodes the α2 subunit of the Na+/K+-ATPase pump on chromosome 1; and the FHM3 (SCN1A) encodes the α1 subunit of the neuronal voltage-gated Nav1.1 channel on chromosome 2. Large genome-wide association studies have identified 13 susceptibility gene variants for migraine with and without aura, three of which regulate glutaminergic neurotransmission, and two of which regulate synaptic development and plasticity. These findings explain the concept of generalized neuronal hyper excitability of the migraine brain.Migraine brain is altered structurally and functionally, secondary to the repetitive state of headache, explains the progression of disease [42,43].
In a tension-type headache, primarily, pericranialmyofascial pain sensitivity is increased. Sensitization of second-order neurons at the level of the spinal dorsal horn or trigeminal nucleus, sensitization of supraspinal neurons, and decreased descending inhibition from supraspinal structures play a major role. NO-related central sensitization may be an important common denominator in pain mechanisms of TTH and while nociception from myofascial tissues is considered important in TTH, the role of a peripheral mechanism as an inciting factor is not clearly known, and certainly, central factors are important for CTTH because general hypersensitivity to pain stimuli has been demonstrated. In a magnetic resonance imaging voxel-based morphometry study, patients with CTTH demonstrated significant gray matter decreases in areas of the brain involved in nociceptive transmission [43-47].In secondary headache, nociceptive receptors are activated by an underlying pathology [42].
5. Co-Morbidities
Epidemiological studies have established a strong association between primary headaches and psychiatric disorders and the reported rates ranged between 56.4-70.3% in migraine and 36.4-77.8 % in TTH [48-52]. In one study, 29.6% of CDH patients met criteria for at least one current psychiatric diagnosis, and 34.9% met criteria for at least one-lifetime psychiatric diagnosis [53]. Depressive and anxiety disorders being the common disorders, other being dysthymia, phobic disorder, obsessive compulsive disorder, somatoform disorders, sleep difficulties and disorders (insufficient and poorer sleep quality, co-sleeping with parents, anxiety related to sleep, night waking, nightmares, bedwetting and pavor), suicidal ideation and an increased risk of suicidality [50-52,54-61].The lifetime prevalence of major depression was threefold higher in persons with migraine and in persons with a severe headache compared with controls[62].TTH is associated with fewer close friends and had a higher rate of divorced parents [63]. Previous studies have shown a significant negative correlation between total intelligence quotient, verbal intelligence quotient, performance intelligence quotient and the frequency of headache attacks[64].
Neurological disorders associated with a headache and migraine, are mainly epilepsy, ADHD and Tourette syndrome [65].The prevalence of epilepsy in patients with migraine varies from 1 to 17%, with an average of 5.9%, and greatly exceeds that of the general population (0.5–1%) . The risk of migraine is more than twice as high in subjects with epilepsy both in probands and in relatives, compared to people without epilepsy [66].The most obvious association is between migraine with aura (MWA) and epilepsy, with a high prevalence of MWA (30.4%) than other types of primary headache in children with seizures [67]. The frequency of migraine in Tourette syndrome subjects was nearly fourfold more than the general population[68].
A migraine is associated with motion sickness, recurrent limb pain, and red ear syndrome, characterized by intermittent unilateral ear pain or burning with redness of that ear that may be associated with headache [69].
Common genetic and environmental risk factors may underlie both migraine and psychiatric disorders . The mechanisms underlying the association between headache and neuro- psychiatric co-morbidities are poorly understood, but both pathophysiological (e.g., serotonergic dysfunction, hormonal influences, shared mechanism in structures of the central nervous system between pain and affective disorders, perhaps involving Limbic activation, dysregulation of the hypothalamic-pituitary-adrenal axis) and psychological (eg, interoceptive conditioning, fear of pain, anxiety sensitivity, avoidance behavior) factors are implicated. [70,71].
Obesity is significantly correlated with a chronic headache, chronic TTH, and migraine. Both diseases show a multifactorial etiology: genetic factors, inflammatory mediators and common lifestyle factors. A dysregulation of the hypothalamic neuropeptides involved in migraine can induce an alteration in feeding regulation. Also, adipokines produced by hypertrophic adipose tissue creates a systemic chronic inflammation that may contribute to the neurogenic inflammation of migraine. Reduction in BMI will lead to a reduction in headache frequency and better outcomes[72].
Other medical problems associated with childhood headaches include atopic disorders (asthma, rhinitis or eczema), hay fever, frequent ear infections, anemia, hyperactive/impulsive behaviour, learning disabilities, stuttering, abdominal illnesses and bowel disease, enuresis,celiac disease, early menarche, and cardiovascular disease, especially ischemic stroke and patent foramen ovale (PFO) [73-78].
6. Investigations
Headache evaluation should include detailed history followed by detailed general and neurological examinations. It should include family history of headaches or any other neurologic, psychiatric or chronic illness, developmental history, injuries, dietary habits of early childhood, school experiences, history of substance and sexual abuse, family relationships, socioeconomic and psychosocial status both of the child and parents and evaluation of any potential co-morbid conditions [79]. A thorough review of lifestyle practices, including caffeine intake, regularity of meals, sleep habits, and exercise, is important in identifying possible sources of modification. It has been suggested that child-completed diaries and teacher observation forms should be used more widely [80]. An appropriate headache diary should be kept to document the headache characteristics and associated symptoms, frequency, duration, degree of disability and the use of medications. Headache severity should be quantified using a pain rating scale, visual analogue scale or other equivalents according to age. Disability assessment should include the impact on school, home, and social activities and can be assessed with tools such as PedMIDAS [81].
Primary headaches comprise migraine, TTH, cluster headache, other autonomic cephalgias and other primary headache disorders, are diagnosed according to the International Classification of Headache Disorders, 3rd edition (beta version). In secondary headache disorders, headache is the symptom of identifiable structural, metabolic or other abnormality.
There is often difficult to make the diagnosis of primary headache disorders in children because of frequent headache transformations, changes in phenotype with age and sex, coexisting migraine and TTH, communication barriers, not fulfilling the prior episode or duration criteria requirement, and lack of a biological-based classification system for TTH and migraine Typical features of migraine may not develop until later in life. Photophobia and phonophobia often do not develop until after age 12.Younger children have a more difficult time describing pain [82]. Important clues can include paroxysmal events where the child appears unwell or pale, vomits, or bangs or holds his or her head.
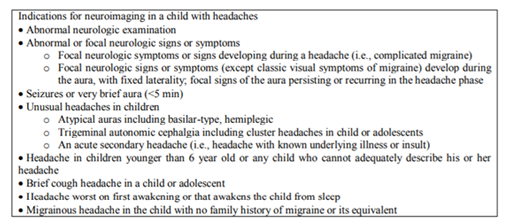
Almost all type of headache syndromes affecting adults are being described in young patients [83]. Neuro imaging is warranted when the neurologic examination is abnormal or unusual neurologic features occur during the migraine (Table 1). MRI is the imaging of choice. The rate of abnormalities found on standard imaging for headache is between 14% and 28% [84]. The ophthalmic examination is necessary to look for papilledema. The electroencephalogram and neurophysiological examinations (VEP, event related potentials, EMG) are of limited value in the routine evaluation of headaches except from “migraine triggeredseizures”[85,86].
7. Treatment
Treatment of headache requires correct and timely diagnosis followed by a comprehensive treatment program, including acute and preventive treatment and a biobehavioral plan [87].
Acute treatment, for stopping a headache attack on a consistent basis with return to functions as soon as possible with the goal being 2 hours maximum, includes 2 groups of medicines: nonsteroidal anti-inflammatory drugs (NSAIDs) and triptans. Ibuprofen is the effective and preferred drug for children older than 6 years. Acetaminophen can be effective in those with a contraindication to NSAIDs. Naproxen sodium and aspirin are other options.
Currently 2 triptans are approved by the FDA for the treatment of migraine in pediatric population. Almotriptan is approved in age 12-17 years and rizatriptan is approved in age 6-17 years. Controlled clinical trials demonstrate that intranasal sumatriptan is safe and effective in adolescent (>12 years of age) with migraine. Zolmitriptan has shown efficacy in adolescents. Triptans vary by rapidity of onset and biological half-life related to their lipophilicity and dose. Clinically, 60-70% of patients respond to the first triptans tried, with 60-70% of the patient who didn‟t respond to the first triptans tried, responding to next triptan. Therefore, in the patient who doesn‟t respond to the first triptan in desired way, it is worthwhile to try a different triptan. Studies showed combination of naproxen sodium and sumatriptan may be effective in children. Recently, FDA approved combination of sumatriptan and naproxen sodium for the treatment of acute migraine attacks in pediatric patients above 12 years of age [88].
As a general rule, all acute drug therapy should be combined with rest, sleep and adequate fluid hydration. The most effective way to administer acute treatment is to use NSAIDs first in mild to severe cases, restricting their use to not more than 2-3 times per week, and adding the triptan for moderate to severe, or attacks that have failed NSAIDs use, restricting to not more than 4-6 attacks per month. Overuse needs to be avoided to prevent the transformation of the migraines into the MOH. The most effective way to administer acute treatment is to use NSAIDs first in mild to severe cases, restricting their use to not more than 2-3 times per week, and adding the triptan for moderate to severe, or attacks that have failed NSAIDs use, restricting to not more than 4-6 attacks per month. Overuse needs to be avoided to prevent the transformation of the migraines into the MOH. For acute treatment of nausea and vomiting with headache, antiemetics with dopamine antagonism (prochlorperazine and metoclopramide) have the best efficacy and are very effective when combined with ketorolac and intravenous fluid in the emergency department for treatment of an intractable headache. Subcutaneous sumatriptan can also be used. When they are not effective, further inpatient treatment of severe intractable headache or status migrainous may require dihydroergotamine. Sodium valproate is used when dihydroergotamine is contraindicated or has been ineffective. Dihydroergotamine should not be given in the 24 hours after triptan use. Triptans are contraindicated in patient treated with ergotamine within 24 hours and within 2 weeks of treatment with monoamine oxidase inhibitors. Both triptans and ergotamine are contraindicated in hemiplegic migraine [89].
Preventive treatment needed, if headaches are frequent (>1 headache/ week) or there is more than one disabling headache/month (missing school, home, or social activities, or a PedMIDAS score >20) with the goal to reduce frequency (1-2 headaches or fewer per month) and disability (PedMIDAS score < 10). Prophylactic agents should be given in adequate dose for at least 4-6 months and then weaned over several weeks . Among all preventive medications only flunarizine demonstrated a level of effectiveness viewed as substantial [87]. The most commonly used preventive therapy for headache and migraine is amitriptyline. Other prophylactic medicines are topiramate, valproic acid, levetiracetam, gabapentin, lamotrigine,zonisamide, pregabalin, cyproheptadine, and propranolol [90]. Among these, only topiramate is approved by FDA, in adolescents 12-17 years of age [91].Neutraceuticals including riboflavin, coenzyme Q10, butterbur and magnesium showed some efficacy [92-94]. Onabotulinumtoxin A is FDA-approved medication for a chronic migraine in adults and showed improvement in disability scores and headache frequency in pediatric chronic daily headache patients and in pediatric chronic migraine [95].
Most common drugs used in the management of migraine in children and adolescents are summarized in Table 2. Same general principles and medications are used in TTHs. Flupiritine is a nonopioidanalgesic that has been approved in Europe for the treatment of TTH in children as young as age 6 years. Amitriptyline has the most evidence of effective prevention of TTH. Melatonin also showed some efficacy [96].
Drug treatment should be selected for each patient according to his or her need and expected response to it and limited by their side effects. If prophylaxis fails review the diagnosis and for the compliance with treatment, medication overuse, and comorbidities.
A biobehavioral plan includes a discussion of adherence, elimination of barriers to treatment, avoidance of potential triggers (skipping meals, dehydration, decreased or altered sleep) andhealthy habit management (adequate fluid intake without caffeine, regular exercise, not skipping meals, and making healthy food choices, and adequate (8-9 hours) sleep on a regular basis) to cope with both the acute attacks and frequent or persistent attacks if present . Biofeedback-assisted relaxation and cognitive behavioral therapy are effective for both acute and preventive therapy . Physiotherapy may be appropriate for musculoskeletal symptoms. Yoga, meditation and acupuncture also said to have some benefit .
The management of secondary headache depends on the cause. MOH, defined as a headache present for more than 15 days/month for longer than 3 months and intake of a simple analgesic on more than 15 days/month and/or prescription medication including triptans or combination medications on more than 10 days/month. MOH frequently complicate primary and secondary headaches. Signs that raise suspicion of medication overuse are the increasing use of analgesics (non-prescription or prescription) with either decreased effectiveness or frequently wearing off (i.e., analgesic rebound). This can be worsened by using ineffective and under-dose medications or misdiagnosing the headache. It can be managed by withdrawal of overused medication and review and reassessing the underlying primary headache disorder .
Based on the pathophysiology of primary headaches, CGRP blockers and receptor antagonists, 5-HT1F agonist, glutamate antagonists, selective neuronal and inducible nitric oxide (NO) synthase inhibitors, NO-cGMP pathway blockers, and drugs that target ghrelin, leptin and orexin receptors are under development for both acute and preventive treatment for primary headaches. Therapies that modify brain networks and their functions are being developed. Transcranial magnetic stimulation, supraorbital transcutaneous stimulation, transcutaneous vagal nerve stimulation, transcutaneous stimulation of the auricular branch of the vagal nerve and sphenopalatine ganglion stimulation had also shown efficacy in migraine treatment [42, 97] Surgical options include decompression of various trigger sites are in evolving stage, and involve decompression of supraorbital, supratrochlear, zygomatico-temporal and greater occipital nerve [98].
In long-term follow-up of childhood migraine, up to 1/3rd of patients will experience remission [99]. Another 20-25% shift from migraine to TTH or vice versa [99]. Diagnoses of primary headache subtypes change over time due to overlapping symptoms and possibly related to maturation . Of those who had become parents, 52% have in their present or previous families had one child or more who had developed recurrent headache, probably of the migraine-type .
Risk factors that predict persistence and unfavourable clinical course include headache onset early in life, frequent headaches, increasing time between headache onset and first examination, female sex, maternal history of headache, comorbid psychiatric illness, poor sleep and stress, medication overuse, and poor self- efficacy for managing headaches [101,102].
Outcome research for paediatric migraine headaches is limited, thus restricting knowledge of the effectiveness of long-term management and outcome. However, more than 93% of patients treated with a multidisciplinary team approach described significant improvement in headaches after 5 years[103].
8. Conclusion
Headache disorders represent a major public health problem and have been underestimated,under-recognized and under-treated throughout the world [104]. More long-term comprehensive population-based studies and research studies needed to understand the pathophysiology and exact mechanism as well as in the development of drugs for management of childhood headaches.
References
- Lateef T.M., Merikangas K.R., He J., Kalaydjian A., Khoromi S., Knight E., et al., Headache in a national sample of American children: prevalence and comorbidity, J. Child Neurol. 14(5), 536–543 (2009).
- Kliegman R.M., Stanton B.F., St Geme III J.W. and Schor N.F., Nelson Textbook of Paediatrics, First South Asia edition. New Delhi, India: Reed Elsevier India Pvt. Ltd., 2016, ch. 595, pp. 2863-74.
- Kernick D. and Campbell J., Measuring the impact of headache in children: a critical review of the literature, Cephalalgia. 29(1), 3-16 (2009)
- Linde M., Gustavsson A., Stovner L.J., Steiner T.J., Barre J., Katsarava Z., et al., The cost of headache disorders in Europe: the Eurolight project, Eur. J. Neurol. 19(5), 703-11 (2012)
- Pakalnis A. Butz C., Splaingard D., Kring D. and Fong J. Emotional problems and prevalence of medication overuse in pediatric chronic daily headache, J. Child Neurol. 22(12), 1356-9 (2007)
- Stovner L.J., Hagen K., Jensen R.,Katsarava Z., Lipton R., Scher A., et al., The global burden of headache: a documentation of headache prevalence and disability worldwide, Cephalalgia. 27, 193–210 (2007)
- Classification Committee of the International Headache Society: The International Classification of Headache Disorders, 3rd edition (beta version), Cephalalgia. 33 (9), 629-808 (2013)
- Laurrel K., Larsson B. and Eeg-Olofsson O., Prevalence of headache in Swedish schoolchildren, with a focus on tension-type headache, Cephalalgia. 24, 380–8 (2004)
- Gupta R., Bhatia M.S., Dahiya D., Sharma S., Sapra R., Semalti K. and Dua R.P.S., Recurrent headache in Indian adolescents, Indian J. Pediatr. 76(7), 733-737 (2009)
- Stovner L.J., Hagen K., Jensen R., Katsarava Z., Lipton R., Scher A., et al., The global burden of headache: a documentation of headache prevalence and disability worldwide, Cephalalgia. 27, 193–210 (2007)
- Ozge A., Bugdayci R., Sasmaz T., Kaleaqasi H., Kurto O., Karakelle A., et al., The sensitivity and specificity of the case definition criteria in diagnosis of headache: a schoolbased epidemiological study of 5562 children in Mersin, Cephalalgia. 22, 791–798 (2002)
- Zuckerman B., Stevenson J. and Bailey V., Stomachaches and headaches in a community sample of preschool children, Pediatrics. 79(5), 677–682 (1987)
- Sillanpaa M., Piekkala P. and Kero P., Prevalence of headache at preschool age in an unselected child population, Cephalalgia. 11(5), 239–242 (1991)
- Sillanpaa M., Changes in the prevalence of migraine and other headaches during the first seven school years, Headache. 23(1), 15–19 (1983)
- Carlsson J., Prevalence of headache in schoolchildren: relation to family and school factors, Acta. Paediatr. 85(6), 692–696 (1996)
- Sillanpaa M., Piekkala P., Prevalence of migraine and other headaches in early puberty, Scand. J. Prim. Health Care. 2, 27–32 (1984)
- Ozge A., Termine C., Antonaci F., Natriashvili S., Guidetti V. and Wober-Bingol C., Overview of diagnosis and management of pediatric headache. Part I: diagnosis, J. Headache Pain. 12(1),13-23 (2011)
- Abu-Arafeh I., Razak S., Sivaraman B. and Graham C., Prevalence of headache and migraine in children and adolescents: a systemic review of population-based studies, Dev. Med. Child Neurol. 52(12), 1088-97 (2010).
- Wober-Bingol C., Epidemiology of migraine and headache in children and adolescents, Curr. Pain Headache Rep. 17(6), 341 (2013)
- Malik A.H., Shah P.A. and Yaseen Y., Prevalence of primary headache disorders in school- going children in Kashmir valley (north-west India), Ann. Indian Acad. Neurol. 15(1), 100-103 (2012)
- Bille B., A 40-year follow-up of school children with migraine, Cephalalgia. 17(4), 488-91 (1997)
- Virtanen R., Aromaa M., Rautava P., Metsahonkala L., Anttila P., Helenius H., et al., Changes in headache prevalence between pre- school and pre-pubertal ages, Cephalalgia22, 179–185 (2002)
- Zwart J.A., Dyb G., Holmen T.L., Stovner L.J. and Sand T., The prevalence of migraine and tension-type headaches among adolescents in Norway. The Nord-Trøndelag Health Study (Head-HUNT-Youth), a large population-based epidemiological study, Cephalalgia. 24(5), 373- 379 (2004)
- Anttila P., Metsahonkala L. and Sillanpaa M., Long–term trends in the incidence of headache in Finnish schoolchildren, Paediatrics. 117(6),1198-201 (2006)
- Mishra D., Sharma A., Juneja M. and Singh K., Recurrent headache in pediatric outpatients at a public hospital in Delhi, Indian Pediatr. 50, 775-778 (2013)
- Russell M.B., Ostergaard S., Bendtsen L. and Olesen J., Familial occurrence of chronic tension-type headache, Cephalalgia. 19, 207– 210 (1999)
- Friedman A.P., von Storch T.J. and Merritt H.H., Migraine and tension headaches: a clinical study of two thousand cases, Neurology. 4, 773–788 (1954)
- Lance J.W. and Anthony M., Some clinical aspects of migraine. A prospective survey of 500 patients, Arch. Neurol. 15, 356–361 (1966)
- Russell M.B., Levi N., Saltyte-Benth J. and Fenger K., Tension-type headache in adolescents and adults: a population based study of 33, 764 twins, Eur. J. Epidemiol. 21, 153–160 (2006)
- Puca F., Psychological and social stressors and psychiatric comorbidity in patients with migraine without aura from headache centers in Italy:A comparison with tension type headache patients, J. Headache Pain. 1, 7-25 (2000)
- Celle M.E., Carelli V. and Fornarino S., Secondary headache in children, Neurol. Sci. 31(1), 81-82 (2010)
- Kernick D., Rainhold D. and Campbell J. L., Impact of headache on young people in a school population, Br. J. of Gen. Pract. 59, 678-81 (2009)
- Vos T., Flaxman A.D., Naghavi M., Lozano R.,Michaud C., Ezzati M., et al., Years lived with disability(YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systemic analysis for the Global Burden Disease Study 2010, Lancet. 380, 2163-96 (2012)
- Sasmaz T., Bugdayci R., Ozge A., Karakelle A., Kurt O. and Kaleagasi H., Are parents aware of their schoolchildren‟s headache?, Eur.J. Public Health. 14(4), 366–368 (2004)
- Babineau S.E. and Green M.W., Headaches in children, Continuum Lifelong Learning Neurol.18(4), 853–868 (2012)
- Colombo B., DallaLibera D., De Feo D., Pavan G., Annovazzi P.P. and Comi G., Delayed diagnosis in pediatric headache: an outpatient Italian survey, Headache. 51(8), 1267-1273 (2011)
- Akyol A., Kiylioglu N., Aydin I., Erturk A.,Kaya E., Telli E., et al., Epidemiology and clinical characteristics of migraine among school children in the Menderes region, Cephalalgia. 15, 781–787 (2007)
- Lipton R.B., Manack A., Ricci J.A., Chee E.,Turkel C.C. and Winner P., Prevalence and burden of chronic migraine in adolescents: results of the chronic daily headache in ddolescents study (C-dAS), Headache. 51(5), 693-706 (2011)
- Lipton R.B., Bigal M.E., Diamond M., Freitaq F., Reed M.L., Stewart F., AMPP Advisory Group, Migraine prevalence, disease burden, and the need for preventive therapy, Neurology.68(5), 343-9 (2007)
- KristoffersenE.S. andLundqvist C., Medication-overuse headache: a review, J, Pain res. 7, 367-378 (2014)
- Walker H.K., Hall W.D and Hurst J.W.,Clinical methods: the history, physical, and laboratory examinations, 3rd ed. Boston: butterworths, 1990, ch. 54. https:// www.ncbi.nlm.nih. gov/ books/NBK377/
- pathophysiology, J. Neurosci. 35(17), 6619-6629 (2015)
- Ashina S., Bendtsen L. and Ashina M., Pathophysiology of migraine and tension-type headache, Techniques in regional anesthesia and pain management. 16(1), 14-18 (2012)
- Ashina M., Bendtsen L., Jensen R. and Olesen J., Nitric oxide-induced headache in patients with chronic tension-type headache, Brain. 123, 1830–1837 (2000)
- Bendtsen L., Jensen R. and Olesen J., Decreased pain detection and tolerance thresholds in chronic tension-type headache, Arch. Neurol. 53, 373–376 (19960
- Schmidt-Wilcke T., Leinisch E., Straube A., Kampfe N., Draqanski B., Diener H.C., et al., Gray matter decrease in patients with chronic tension type headache, Neurology. 65, 1483– 1486 (2005)
- Monteith T.S. and Sprenger T., Tension type headache in adolescence and childhood: where are we now?, Curr. Pain Headache Rep. 14(6), 424-430 (2010)
- Singh A.K., Shukla R., Trivedi J.K. and Singh D., Association of psychiatric co-morbidity and efficacy of treatment in chronic daily headache in Indian population, J. Neurosci. Rural Pract. 4(2), 132-139 (2013)
- Puca F., Guazzelli M., Sciruicchio V., Libro G., Sarchielli P., Russo S., et al., Psychiatric disorders in chronic daily headache: Detection by means of the SCID interview, J. Headache Pain. 1, 33-7 (2000)
- Corchs F., Mercante J.P., Vera Z., Guendler V.Z., Vieira D.S., Masruha M.R., et al., Phobias, other psychiatric co-morbidities and chronic migraine, Arq. Neuropsiquiatr. 64, 950-3. (2006)
- Guidetti V., Galli F., Fabrizi P., Giannantoni A.S., Napoli L., Bruni O., et al., Headache and psychiatric comorbidity: Clinical aspects and outcome in an 8-years follow up study, Cephalagia. 18, 455-62 (1998)
- Antonaci F., Nappi G., Galli F., Manzoni G.C., Calabresi P. and Costa A., Migraine and psychiatric comorbidity: a review of clinical findings, J. Headache Pain. 14, 115–125 (2011)
- Slater S.K., Kashikar-Zuck S.M., Allen J.R.,LeCates S.L., Kabbouche M.A., O‟Brien H.L., Hershey A.D. and Powers S.W., Psychiatric comorbidity in pediatric chronic daily headache, Cephalalgia. 14(15), 1116–1122 (2012)
- Bruijn J., Locher H., Passchier J., Dijkstra N. and Arts W.F., Psychopathology in children and adolescents with migraine in clinical studies: a systematic review, Pediatrics. 14, 323–332 (2010)
- Pavone P., Rizzo R., Conti I., Verrotti A., Mistretta A., Falsaperla R., Pratico A.D., Grosso G. and Pavone L., Primary headaches in children: clinical findings on the association with other conditions, Int. J. Immunopathol. Pharmacol. 14(4), 1083–1091(2012)
- Smitherman T.A., Kolivas E.D. and Bailey J.R., Panic Disorder and migraine: comorbidity, mechanism, and clinical implications, Headache. 14, 23–45 (2013)
- Seshia S.S., Wang S.J., Abu-Arafeh I. and Hershey A.D., Chronic daily headache in children and adolescents: a multi-faceted syndrome, Can. J. Neurol. Sci. 37(6), 769-778 (2010)
- Miller V.A., Palermo T.M., Powers S.W., Scher M.S., and Hershey A.D., Migraine headaches and sleep disturbances in children, Headache. 14, 362–368 (2003)
- Barbas G., Ferrari M. and Mattews W.S.,Childhood migraine and somnambulism, Neurology. 14, 948–949 (1983) [
- Wang S.J., Fuh J.L., Juang K.D. and Lu S.R., Migraine and suicidal ideation in adolescents aged 13 to 15 years, Neurology. 72(13), 1146-1152 (2009)
- Bellini B., Arruda M., Cescut A., Saulle C., Persico A., Carotenuto M., et al., Headache and comorbidity in children and adolescents, J. Headache Pain. 14(1), 79 (2013)
- Breslau N., Schultz L.R., Stewart W.F., Lipton R.B., Lucia V.C. and Welch K.M., Headache and major depression: Is the association specific to migraine?, Neurology. 54, 308–13 (2000)
- Karwautz A., Wober C., Lang T., Bock A/, Wagner-Ennsqraber C. and Vesley C., Psychosocial factors in children and adolescents with migraine and tension-type headache: a controlled study and review of literature, Cephalalgia. 19(1), 32-43 (1999)
- Parisi P., Verrotti A., Paolino M.C., Urbano A., Bernabucci M., Castaldo R. and Villa M.P., Headache and cognitive profile in children: a cross-sectional controlled study, J. Headache Pain. 11(1), 45-51 (2010)
- Polanczyk G., Lima M.S., Horta B.L., Biederman J. and Rohde L.A., The worldwide prevalence of ADHD: a systematic review and metaregression analysis, Am. J. Psychiatry. 14(6), 942–948 (2007)
- Ottman R. and Lipton R.B., Comorbidity of migraine and epilepsy, Neurology. 14, 2105– 2110 (1994)
- Piccinelli P., Borgatti R., Nicoli F., Calcagno P., Bassi M.T., Quadrelli M., Rossi G., Lanzi G. and Balottin U., Relationship between migraine and epilepsy in pediatric age, Headache. 14(3), 413–421 (2006)
- Kwak C., Vuong K.D. and Jankovic J., Migraine headache in patients with Tourette syndrome, Arch. Neurol. 60(11), 1595-1598 (2003)
- Raieli V., Compagno A., Brighina F., et al., Prevalence of red ear syndrome in juvenile primary headaches, Cephalalgia. 31(5), 597-602 (2011)
- Guidetti V. and Galli F., Psychiatric co-morbidity in chronic daily headache: Pathophysiology, etiology, and diagnosis, Curr. Pain Headache Rep. 6, 492–7 (2002)
- Ferrari M.D., Odnik J., Tapparelli C., Van Kempen G.M., Pennings E.J. and Bruyn G.W., Serotonin metabolism in migraine, Neurology. 39, 1239–42 (1989)
- Farello G., Ferrara P., Antenucci A., Basti C. and Verrotti A., The link between obesity and migraine in childhood: a systemic review, Ital. J. Pediatr. 43. 27 (2017)
- Mortimer M.J., Kay J., Gawkrodger D.J., Jaron A. and Barker D.C., The prevalence of headache and migraine in atopic children: an epidemiological study in general practice, Headache. 14(8), 427–431 (1993)
- Nezu A., Kimura S., Ohtsuhi N., Tanaka M. and Takebayashi S., Acute confusional migraine and migrainous infarction in childhood, Brain Dev. 14, 148–151 (1997)
- Ebinger F., Boor R., Gawehn J. and Reitter B., Ischemic stroke and migraine in childhood: coincidence or causal relation?, J. Child Neurol. 14, 451–455 (1999)
- Anzola G.P., Frisoni G.B., Morandi E., Casilli F. and Onorato E., Shunt-associated migraine responds favorably to atrial septal repair: a case–control study, Stroke. 14, 430–434 (2006)
- Carotenuto M., Esposito M. and Pascotto A., Migraine and enuresis in children. An unusual correlation?, Med. Hypotheses. 75, 120-2 (2010)
- Lionetti E., Francavilla R., Maiuri L., Ruqqieri M., Spina M., Pavone P., et al., Headache in pediatric patients with celiac disease and its prevalence as a diagnostic clue, J. Pediatr. Gastroenterol. Nutr. 49, 202-7 (2009)
- Tietjen G.E., Brandes J.L., Peterlin B.L., Eloff A., Dafer R.M., Stein M.R., et al., Childhood maltreatment and migraine (part I). Prevalence and adult revictimization: a multicenter headache clinic survey, Headache 50(1), 20-31 (2010)
- Lundqvist C., Clench-Aas J., Hofoss D. and Bartonova A., Self-reported headache in schoolchildren: parents underestimate their children‟s headaches, Acta. Paediatr. 95(8), 940–946 (2006)
- Ruangsuwan S. and Sriudomkajorn S., 375 Childhood primary headache: clinical features, the agreement between clinical diagnosis and diagnoses using the International Classification of Headache Disorders in Thai children, J. Med. Assoc. Thai. 90(7), 1309-1316 (2007)
- Hershey A.D., Winner P. and Kabbouche M.A., Use of the ICHD-II criteria in the diagnosis of pediatric migraine, Headache. 45(10), 1288-1297 (2005)
- Cerminara C., Compagnone E., Conigli A., et al., Hypnic headache in children, Cephalalgia. 31(16), 1673-1676 (2011)
- Rho Y.I., Chung H.J., Suh E.S., Lee K.H., Eun B.L., Nam S.O., et al., The role of neuroimaging in children and adolescents with recurrent headaches- multicenter study, Headache. 51(3), 403-408 (2011)
- Oelkers-Ax R., Bender S., Just U., Pfuller U., Parzer P., Resch F., et al., Pattern-reversal visual-evoked potentials in children with migraine and other primary headache: evidence for maturation disorder?, Pain. 108(3), 267–275 (2004)
- Lewis D.W. and Qureshi F., Acute headache in children and adolescents presenting to the emergency department, Headache. 40(3), 200– 203 (2000)
- Lewis D., Ashwal S., Hershey A., Hirtz D., Yonker M. and Silberstein S., Practice parameter: pharmacological treatment of migraine headache in children and adolescents. Report of American academy of neurology quality standards subcommittee and the practice committee of the child neurology society, Neurology. 63(12), 2215-2224 (2004)
- Drugs.com. FDA approves treximet for migraine in teens. Viewed 14 October 2017, https://www.drugs.com/newdrugs/fda-approves-treximet-sumatriptan-naproxen-sodium-migraine-pediatric-patients-4214.html
- O‟Brien H.L., Kabbouche M.A. and Hershey A.D., Treatment of acute migraine in the pediatric population, Curr. Treat Options Neurol. 12(3), 178-185 (2010)
- Hershey AD., Current approaches to the diagnosis and management of paediatric migraine, Lancet Neurol. 9(2) 190-204 (2010)
- Kacperski J., Kabbouche M.A., O‟Brien H.L. and Weberding J.L., The optimal management of headaches in children and adolescents, Ther. Adv. Neurol. Disord. 9 9(1) 53-68 (2016)
- Slater S.K., Nelson T.D., Kabbouche M.A., LeCates S.L., Horn P., Seqers A., et al., A randomized, double-blinded, placebo-controlled, crossover, add-on study of CoEnzyme Q10 in the prevention of pediatric and adolescent migraine, Cephalalgia. 31(8), 897-905 (2011)
- Bruijn J., Duivenvoorden H., Passchier J., Locher H., Dijkstra N. and Arts W.F., Medium-dose riboflavin as a prophylactic agent in children with migraine: a preliminary placebo-controlled, randomised, double-blind, cross-over trial, Cephalalgia. 30(12), 1426-1434 (2010)
- Seshia S.S., Abu-Arafeh I. and Hershey A.D., Tension-type headache in children: the Cinderella of headache disorders!, Can. J. Neurol. Sci. 36(6), 687-695 (2009)
- Chan V.W., McCabe E.J. and MacGregor D.L., Botox treatment for migraine and chronic daily headache in adolescents, J. Neurosci. Nurs. 41(5), 235-243 (2009)
- Miano S., Parisi P., Pelliccia A., Luchetti A., Paolino M.C. and Villa M.P., Melatonin to prevent migraine or tension-type headache in children, Neurol. Sci. 14(4), 285–287 (2008)
- Antonaci F., Ghiotto N., Wu S., Pucci E. and Costa A., Recent advances in migraine therapy, Springerplus. 5, 637 (2016)
- Poggi J.T., Grizzell B.E. and Helmer S.D., Conformation of surgical decompression to relive migraine headaches, Plast. Reconstr. Surg. 122(1), 115-122 (2008)
- Kienbacher C., Wober C., Zesch H.E., Hafferl-Gattermayer A., Posch M., Karwautz A., et al., Clinical features,classification and prognosis of migraine and tension typeheadache in children and adolescents: a long-term follow-up study, Cephalalgia26, 820–830 (2006).
- Ozge A., Sasmaz T., Cakmak S.E., et al., Epidemiological-based childhood headache natural history study: after an interval of six years, Cephalalgia. 30(6), 703-712 (2010)
- Laurell K., Larsson B., Mattsson P. and Eeg-Olofsson O., A 3-year follow-up of headache Idiagnosis and symptoms in Swedish schoolchildren, Cephalalgia. 26(7), 809-815 (2006)
- Probyn K., Bowers H., Caldwell F., Mistry D., Underwood M., Matharu M., et al., Prognostic factors for chronic headache. A systemic review, Neurology. 89(3), 291-301 (2017)
- Kabbouche M.A., Powers S.W., Vockell A.B., LeCates S.L., Ellinor P.L., Segers A., et al., Outcome of a multidisciplinary approach to pediatric migraine at 1, 2, and 5 years, Headache. 45(10), 1298-1303 (2005)
- Diamond S., Silberstein S., Loder E., Reed M., Bigal M.E. and Lipton R.B., Patterns of diagnosis and acute and preventive treatment in migraine in the United States. Results of the American Migraine Prevalence and Prevention Study, Headache. 47(3), 355-63 (2007).