Information
Journal Policies
Evaluation of Anterior Segment Changes Following Laser Peripheral Iridotomy by Scheimpflug Imaging
Ahmed Aboleila1, Hossam El-Sharkawy1, Ayman Abd El Ghafar A1, Asaad A. Ghanem1*
Copyright : © 2018 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Purpose : To assess the effect of LASER peripheral iridotomy on anterior segment parameters using the Scheimpflug imaging system in the management of angle closure glaucoma.
Design : Cross-sectional, prospective interventional study.
Participants : A total of 31 eyes with of 28 patients diagnosed to have primary narrow angle or angle closure glaucoma.
Methods : All participants had LASER peripheral iridotomy to control IOP. Anterior chambers of the treated eyes were then evaluated using Scheimpflug images measuring different parameters of anterior chamber and angles. Main outcome measures include evaluation of anterior chamber depth at different points (central & peripheral at 4 mm & 8 mm from center) and anterior chamber angle at 4 quadrants.
Results : Mean Angular width had an average in degrees as following; superior mean angular width at 0º (22.99º), at 90º (23.01º), at 180º (23.94º), and at 270º was 23.12º before LPI. After LPI they increased to 25.8º, 25.9º, 26.24º, and 25.9º respectively. Mean pre LPI IOP was 19.48 (±4.42 mmHg) with 9 out of 31 eyes on topical medication whereas following LPI, mean IOP was 16.31 (±2.97 mmHg). LPI significantly increased PACD immediately and on intervals post iridotomy results stabilized but there was no influence on central ACD (p=>0.05). Significant PACD deepening and widening of ACA were observed with negligible change in CACD.
Conclusions : PACS patients had more significant change in PACD at both 4 mm and 8 mm circles and ACA at all quadrants in comparison to both PAC and ACG patients. The Scheimpflug imaging system can evaluate PACD and ACA non-invasively and easily and all parameters of the anterior ocular segment automatically, and has high reliability.
Anterior Segment, Laser Peripheral Iridotomy, Scheimpflug Imaging,Ophthalmology
1. Introduction
Angle-closure glaucoma (ACG) is a major form of glaucoma. It is a leading cause of blindness world- wide. Laser peripheral iridotomy (LPI) is the standard first-line intervention in both acute and chronic forms. It is successful in preventing recurrence of acute attacks and virtually eliminates the risk of an acute attack in the fellow eye. Peripheral iridotomy eliminates papillary block, allowing the convex iris to flatten and widening the anterior chamber angle. Such changes in anterior chamber angle morphology are difficult to be assessed. Gonioscopy is difficult to be performed in are producible fashion, limiting the ability to quantify changes after LPI[1].
Ultrasound biomicroscopy (UBM) gives reproducible images of the cross-sectional anterior chamber angle anatomy, with very high resolution. Enhanced depth imaging and swept- source anterior segment optical coherence tomography (AS-OCT). Both help provide higher resolution imaging of the anterior chamber and even help in evaluating the in vivo micro architecture of the trabecular outflow pathway[2].
The Scheimpflug system is a rotating camera that noninvasively assesses the anterior segment of the eye. It takes about 2 seconds to generate an image of the anterior eye. The instrument acquires cross sectional images o f the anterior segment. Information from numerous data points is used to calculate corneal thickness, corneal curvature, anterior chamber angle and depth, from the height data it generates from the imaged points. This gives reproducible information for anterior chamber biometry[3].
In this study, we aimed to know the effects of peripheral laser peripheral iridotomy (LPI) on peripheral anterior chamber depth (PACD), central internal anterior chamber depth (CACD) and anterior chamber angle (ACA) using Scheimpflug imaging system.
2. Materials And Methods
This was a prospective, non-randomized, non-comparative, interventional study. Thirty one (31) eyes of 28 patients with primary angle closure (PAC) were enrolled from Glaucoma Clinic of Mansoura Ophthalmic Center, Mansoura, Egypt. The inclusion criteria included primary angle closure suspects PACS and the eyes with either PAC or ACG where LPI was done prophylactically or to help lower IOP. All eyes with acute angle closure, cataract, open angles, previous lasers or surgery were excluded.
Primary angle closure (PAC) was defined as raised IOP (>21 mm Hg) associated with non-visibility of the filtering trabecular meshwork for more than 180 degrees on gonioscopy, in the presence of peripheral anterior synechiae or iris atrophy or glaucomflecken without disc damage, or field changes.
Other causes of synechiae were considered ACG patients. Gonioscopic grading of the angle was performed in a darkened room with standardized, minimum-possible slit- lamp illumination. The Sheimpflug camera scanned the anterior eye segment with 64 image acquisition scan protocol at total of 40,960 points ranging from the optical axis to the limbus. It automatically evaluated the PACD, central ACD and ACA (After identifying related points) and the values for PACD used included PACD at 4 mm and 8 mm at 3'0/9'0/12'0/6'0 clock hours. The LPI was done according to a standard protocol using Nd: YAG Laser. The data for PACD, ACD, ACA was analyzed pre-iridotomy immediately post iridotomy and at 1 day, 1 week and 1 month post iridotomy.
Data analysis was performed by commercially available software SPSS v16 (Chicago, Illinois, USA). Paired t-test was used to evaluate the differences. Reproducibility of outcome of LPI was determined by 95% confidence interval. A level of P≤ 0.05 was considered significant.
Data were collected in a master sheet, coded, entered and analyzed using EPI-INFO.4 Data were presented as Mean ± Standard deviation for quantitative variables & number and percentage for qualitative variables.
3. Results
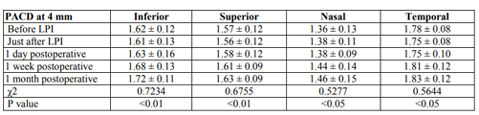
Thirty one eyes of 28 patients were enrolled and seen at 1-day, 1-week, and 1 month follow-up intervals: 13 males and 15 females. Mean age was 60.97 years (±6.595; range, 45-75 years), mean spherical equivalent refraction +0.5 diopters (D) (±1.14 D; range, “1.5 D to +3.5 D). Best Corrected Visual Acuity was 0.82 (20/25) ±0.32. Mean Angular width had an average in degrees as following; superior mean angular width at 0º (22.99º), at 90º (23.01º), at 180º (23.94º), and at 270º was 23.12º before LPI. After LPI they increased to 25.8º, 25.9º, 26.24º, and 25.9º respectively. Mean pre LPI IOP was 19.48(±4.42 mmHg) with 9 out of 31 eyes on topical medication whereas following LPI, mean IOP was 16.31(±2.97 mmHg). LPI significantly increased PACD immediately (Table 1) and on intervals post iridotomy results stabilized but there was no influence on central ACD (p=>0.05). Significant PACD deepening and widening of ACA were observed.
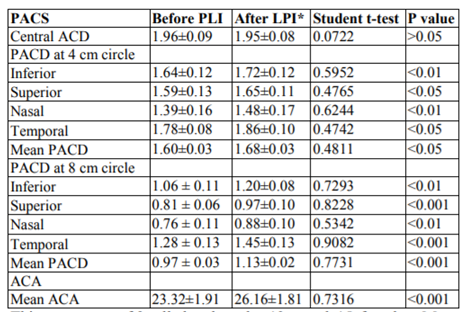
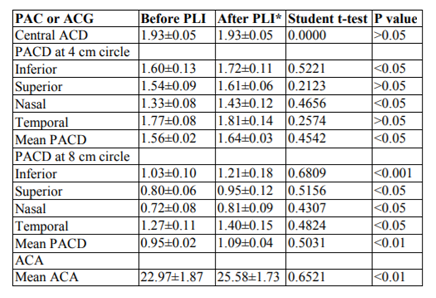
Later on subjects were divided into two groups; group 1 included PACS (Table 2) and group 2 included PAC and ACG patients (Table 3). Group 1 showed better response to LPI.
4. Discussion
In the past, there were several studies that measured ACD quantitatively. However, the instruments or methods employed are not widely used today because of expensiveness, handling difficulty, complicated quantitative analysis, and low reproducibility. Those methods are not suitable for screening eyes that are at a high risk of developing PACG or CACG. Some optical methods have been introduced for evaluating PACD. However, these methods also have many disadvantages.
In the present study, we measured ACD at center, 4 mm and 8 mm circles which center is the cornea apex. However, Li et al. (2014) defined PACD as 4 mm from cornea apex and this was more far away from the iridotomy site.
In group I (PACS), ACA increased significantly in all quadrants with about 2.84 degrees. PACD in 4 mm circle showed significant increase in all 4 quadrants with about 0.08 mm while at 8 mm circle with about 0.16 mm.
In group II (PAC or ACG), ACA increased significantly in all quadrants with about 2.61 degrees. PACD 4 mm circle showed significant increase in all 4 quadrants with about 0.08 mm while at 8 mm circle with about 0.14 mm.
The results in the present study, showed insignificant change in central anterior chamber depth (CACD) after the LPI, agreeing with Esmaeili et al. (2013) and Jain et al. (2013) studies. The LPI flattens the iris and pushes it toward the posterior chamber; however, the location of the lens is not affected; thus not affecting the CACD.
In the present study PD might affect the anterior chamber parameters measurement. In order to overcome this problem, we tried to control the surrounding lights and fixation to ensure that PD remained almost unchanged in all measurements sessions.
Similar to our study, Esmaeili et al. (2013) reported that the mean ACA increased significantly from 25.59 ± 4.41 degrees to 26.46 ± 4.33 degrees (P=0.009). However, the rise in the CACD was not statistically significant (P=0.09).
Also Vryonis et al. (2013) reported that post-LPI, the average ACD increased from 1.88 ± 0.36 to 1.93 ± 0.32 mm (P=0.49). The average ACA widened from 21.1 ± 4.8 to 23.4 ± 3.8 degrees (P=0.01).
Another study held by Talajic et al. (2013) agreed with our study. It concluded that ACA increased significantly from 26.7 ± 0.9 degrees to 28.2 ± 0.8 degrees (P < 0.001). CACD increased slightly after LPI, but this was not statistically significant from 2.13 ± 0.05 mm to 2.15 ± 0.05 mm (P =0.109).
On the contrary, Li et al. (2014) found that there were statistically significant differences before and one week after LPI. All nasal and temporal ACA and PACD (all P < 0.05) showed significant change except for superior ACA (P = 0.053) and inferior ACA (P = 0.389), CACD (P = 0.453) and PD (P = 0.221).
As opposed to the present study which showed presistant significant increase in PACD in all quadrants all over the study even with slight decrease in significant at end of study. Grewal et al. (2008) observed that there was no significant changes in deepening of PACD at 4 mm at any quadrant neither immediately or 1 week post LPI as changes was as follow in mm; inferiorly before LPI was 1.63 mm and didn’t change as it remained 1.63 mm immediately after LPI then 1.72 mm on 1 week post LPI, superiorly 1.51 mm to 1.53 mm then 1.62 mm, nasaly 1.45 mm to 1.45 mm then 1.52 mm, and temporally from 1.75 mm to 1.72 mm then 1.8 mm, but at 8 mm significant changes was observed at immediate follow-up then after 1 week changes decreased till there was no significance as follow in mm; inferiorly from 0.95 mm to 1.23 mm then 1.07 mm at 1 week post LPI, superiorly 0.73 mm to 1.07 mm then 0.87 mm, nasaly from 0.65 mm to 0.88 mm then 0.71 mm and temporally from 1.18 mm to 1.38 mm to 1.32 mm but there was a similarity with this study in that there was no significant changes in CACD all over study.
The Scheimpflug imaging system can evaluate PACD and ACA non invasively and easily and all parameters of the anterior ocular segment automatically, and has high reliability. The advantages of this system are mechanical simplicity, quick, non invasive, ease of handling, objectivity, and good quantitative measurement. This system may be useful for detecting eyes with narrow angles and at risk for developing acute attacks during regular eye checkup.
References
- Grewal SP, Jain R, Grewal D. Evaluation of anterior segment changes following LASER peripheral iridotomy using pentacamScheimpflug imaging system in eyes with primary angle closure (PAC). Highlights of Ophthalmology 2008; 36(4):13-14.
- Friedman D and He M. Anterior chamber angle assessment techniques. SurvOphthalmol 2008; 3:250-73.
- Esmaeili A, Barazandeh B, Ahmadi S, Haghi A, AhmadiHosseini SM, Abolbashari F. Assessment of the anterior chamber parameters after laser iridotomy in primary angle close suspect using Pentacam and gonioscopy. Int J Ophthalmol 2013; 6:680-84.
- Dean AG, Dean JA, Colombier D, Brendel KA, Smith DC, Burton AH. Epi Info, version 6: a word processing, database, and statistics program for epidemiology on microcomputers. Atlanta: Centers for Disease Control and Prevention; 1994.
- Li X, Wang Z, Cao Q, Hu L, Tian F, Dai H. Pentacam could be a useful tool for evaluating and qualifying the anterior chamber morphology. Int J ClinExp Med 2014; 7:1878-82.
- Jain R, Grewal D, Grewal SP. Quantitative analysis of anterior chamber following peripheral laser iridotomy using Pentacam in eyes with primary angle closure. Eur J Ophthalmol 2013; 23:55-60.
- Vryonis N, Nikita E, Vergados I, Theodossiadis P, Filippopoulos T. Anterior chamber morphology before and after laser peripheral iridotomy determined by Scheimpflug technology in white patients with narrow angles. J Glaucoma 2013; 22:679-83.
- Talajic JC, Lesk MR, Nantel-Battista M, Harasymowycz PJ. Anterior segment changes after pilocarpine and laser iridotomy for primary angle-closure suspects with scheimpflug photography. J Glaucoma 2013; 22:776-79.