Information
Journal Policies
A Choroidal Lesion with Extrascleral Extension: A Case Report
Christopher D. Conrady1*, Kirk Winward2, Roger Harrie1
2.Retina Associates of Utah, Murray, UT 84107, US.
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Purpose: Choroidal lesions are difficult to discern diagnostically due to their subretinal location and the long differential diagnosis associated with them. As such, any additional features of the lesion noted on examination or further imaging helps in narrowing the differential diagnosis. Standardized A and B -scan ultrasonography is an imaging modality crucial in differentiating variou s intraocular tumors and specific ultrasound features may help narrow the differential diagnosis in the case of choroidal lesions.
Observation: We present the case of a 64-year-old male with a choroidal lesion noted to have extrascleral extension subsequently identified as an isolated low-grade B-cell lymphoma by fine needle aspiration.
Conclusion and importance: In conclusion, identifying extrascleral extension on A- and B-scan ultrasonography of a lesion helps narrow the differential diagnosis to choroidal lymphomas or melanomas and posterior scleritis.
Extrascleral, lymphoma, choroid, tumor,Ophthalmology
1. Introduction
Intraocular B-cell lymphoma is a uveitic masquerader that can involve various intraocular structures such as the vitreous, retina, choroid, iris, ciliary body, and anterior chamber. The location that is primarily involved is suggestive of the aggressiveness, morbidity, and mortality of the lesion. For example, primary vitreoretinal lymphomas are associated with high rates of central nervous system involvement and subsequently high morbidity and mortality, while uveal lymphomas are usually low grade and have a prolonged, benign clinical course[1,2].
Anatomical location of the tumor aside, intraocular lymphomas usually have low to medium internal reflectivity.[3] In the case described herein, we found that extrascleral extension of uveal lymphoma is a critical finding on ultrasonography in identifying the underlying cause of the choroidal mass.
2. Case Report
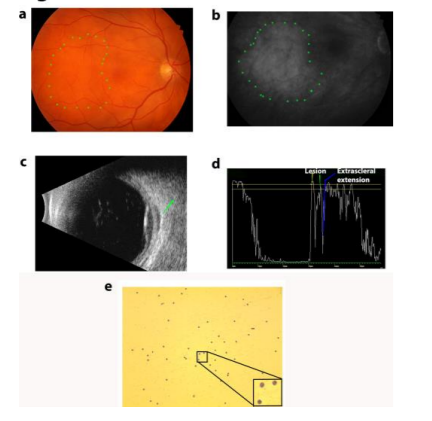
A 64-year-old man with past medical history of medication-treated hypertension and anisometropic amblyopia of the left eye presented with a history of floaters nasally and a months. His best-corrected visual acuity was 20/25 OD and 20/40 OS with a moderately hyperopic refraction worse in his left eye. Intraocular pressures were 15 mmHg OU and external examination was normal bilaterally except for 1+ nuclear sclerosis OU. His fundus examination OS was unremarkable while OD showed an ill-defined amelanotic choroidal lesion temporal to the macula without overlying edema, hemorrhage, or orange pigment (Figure 1a). Fluorescein angiography findings were consistent with fundus photos and a diagnostic ultrasound was performed that further characterized extrascleral extension of the choroidal lesion and estimated the size to be 3mm x 10mm x 10mm (Figure 1b-d).
Due to concern for malignancy and unclear diagnosis from history, imaging, and examination, a fine needle biopsy of the lesion was performed and revealed a CD 20+ lowgrade B cell lymphoma (Figure 1e). To rule out systemic involvement, computed tomography (CT) was performed of the chest, abdomen, and pelvis. Only a small, solitary pulmonary nodule and a mildly enlarged lymph node in the porta hepatis were found on imaging. These imaging findings were not felt to be clinically significant by his primary care provider. Additionally, a body full PET scan was performed and did not show any evidence of systemic lymphoma. Because there is only an isolated choroidal lymphoma, the patient is currently being scheduled for proton beam irradiation of the ocular lesion.
3. Discussion
The nomenclature describing the growth of lymphoid cells in the uveal tract has gone through several changes since the original term ‘uveal lymphosarcoma’ was applied by Triebenstein in 1920. Ryan and Zimmerman coined the term reactive lymphoid hyperplasia in a pathological series published in 1972.[4] This implied a more benign proliferation of lymphoid cells that was felt to lie at one end of a spectrum with aggressive, systemic, non-Hodgkins’ lymphoma at the other end. Several investigators have revisited pathological specimens previously diagnosed as reactive lymphoid hyperplasia and have reclassified most of them as extranodal marginal zone B-cell lymphoma[5].
Uveal lymphoma is a relatively rare condition that often presents with a painless decrease in vision and may be confused with other entities.[6] When the condition presents as multi-focal creamy yellow lesions, the differential diagnosis includes such entities as acute multifocal placoid pigment epitheliopathy (AMPPE), birdshot choroidopathy, and vitreoretinal lymphoma, although Shields found this fundus appearance in only half of their published cases of uveal lymphoma.[7]The differential diagnosis of an amelanotic, isolated choroidal mass as found in our patient includes choroidal hemangioma, amelanotic melanoma, metastatic lesions, osseous choriostoma, and nodular posterior scleritis. The extrascleral component identified on A- and B-scan in this case narrows the diagnostic possibilities to choroidal melanoma or uveal lymphoma with extrasceral extension, or posterior scleritis.
Diagnostic A and B-scan ultrasound is the most sensitive test to document extrascleral extension of choroidal lesions. Both the choroidal and extrascleral components show low to medium reflectivity and spontaneous vascularity.[8] Singh described extrascleral extension in 76% of his patients with ocular lymphoma with concentric scleral and episcleral thickening in 86% of these and a discrete extrascleral mass in 45%.[9] It is felt that this extension is due to the choroidal component of the lymphoma being able to penetrate the sclera through emissary channels.[10] Biopsy is the definitive test to confirm the diagnosis and is recommended in all of these patients when possible. However, there are some patients unable to undergo general anesthesia due to health concerns making ultrasound findings of the lesion critical in the diagnosis.
In uveal lymphoma, systemic evaluation is important, as prior studies have found that 31% of patients with uveal lymphoma had systemic lymphoma at presentation and 22% of these patients were found to have systemic disease only after further evaluation of presumed isolated ocular disease.[11] This was further supported by a report that showed 23% of patients with primary uveal lymphoma had systemic disease discovered on staging studies.[12] The prognosis for most patients with primary uveal lymphoma without a systemic component is excellent with the vast majority of cases remaining confined to the eye.[11] However, the prognosis for intraocular lymphoma with widespread systemic lymphoma is much more variable and depends on subtype of lymphoma and grading and staging at the time of diagnosis.[13]
Patient Consent: Informed consent was obtained from the patient for publication of this case report and any accompanying images. The study in its entirety is HIPAA compliant.
4. Acknowledgements And Dis Clos Ures
Funding:
This work was supported in part by an unrestricted grant from the Research to Prevent Blindness to the University of Utah Department of Ophthalmology.
Conflict of Interest:
The following authors have no financial disclosures: CDC, KW, and RH.
Authorship:
All authors attest that they meet the current ICMJE criteria for Authorship.”
References
- Pe'er J, Hochberg FH, Foster CS. Clinical review: t reat ment of vitreoret inal ly mpho ma. Ocul Immunol Inflamm. Sep-Oct 2009; 17(5):299-306.
- Coupland SE, Foss HD, Hidayat AA, Cockerham GC, Hu mmel M, Stein H. Extranodal marg inal zone B cell ly mpho mas of the uvea: an analysis of 13 cases. J Pathol. Jul 2002; 197(3):333-340.
- Jahnke K, Thiel E, Abrey LE, Neu welt EA, Korfel A. Diagnosis and management of primary intraocular ly mphoma: an update. Clinical ophthalmology. Sep 2007; 1(3):247-258.
- Ryan SJ, Zimmerman LE, King FM. Reactive ly mphoid hyperplasia. An unusual form of intraocular pseudotumor. Transactions - American Academy o f Ophthalmology and Otolaryngology. American Academy of Ophthalmology and Otolaryngology. May-Jun 1972; 76(3):652-671.
- Cockerham GC, Hidayat AA, Bijwaard KE, Sheng ZM. Re-evaluation o f "reactive y mphoid hyperplasia of the uvea": an immunohistochemical and mo lecular analysis of 10 cases. Ophthalmology. Jan 2000; 107(1):151-158.
- Erickson B, Mantopoulos D, Schoenfield L, Cebulla CM. Mult imodal Imaging and Clin icopathologic Correlation in Primary Uveal Ly mpho ma. Case reports in ophthalmology. Jan-Apr 2016; 7(1):39-43.
- Shields JS, CL. Intraocular Tu mo rs: An Atlas and Textbook. 2016:527-534.
- Chang TS, Byrne SF, Gass JD, Hughes JR, Johnson RN, Murray TG. Echographic findings in benign react ive ly mphoid hyperplasia of the choroid. Arch Ophthalmol. Jun 1996; 114(6):669-675.
- Aronow ME, Portell CA, Sweetenham JW, Singh AD. Uveal ly mphoma: clin ical features, diagnostic studies, treatment selection, and outcomes. Ophthalmology. Jan 2014; 121(1):334-341.
- Jusufbegovic D, Kim V, Char DH. Primary uveal ly mphoma with epibulbar extension masquerading as an intraocular in flammation. Can J Ophthalmol. Feb 2015; 50(1):e24-26.
- Mashayekhi A, Shukla SY, Sh ields JA, Shields CL. Choroidal ly mpho ma: clinical features and association with systemic ly mpho ma. Ophthalmology. Jan 2014; 121(1):342-351.
- Singh AD, Seregard, S. Ocular Tu mors. 2016; 7:53-59.
- Hoffman PM, McKelvie P, Hall AJ, Stawell RJ, Santamaria JD. Intraocular ly mpho ma: a series of 14 patients with clin icopathological features and treatment outcomes. Eye (Lond). May 2003; 17(4):513-521.