Information
Journal Policies
Morpheaform Basal Cell Carcinoma – Case Report
Carolina Peres Batalha1,Marcelo Vicente Andrade Sobrinho2,Beatriz Pedrosa Aguiar Casagrande3,Grasiela Melhado Simionato4,Leticia Tavares Selegatto5
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
1. Introduction
Basal cell carcinoma, the most common malignant tumor of the periocular skin, can have several clinically recognized appearances. In contrast with the most common appearance which is a nodulo-ulcerative, firm, elevated, pearly , with telangiectatic vessels, slowly enlarging, that usually have relatively distinct margins, the sclerosing or morpheaform variant of basal cell carcinoma frequently has relatively indistinct margins and does not tend to ulcerate until late.
In neglected cases, extensive areas of ulceration called “rodent ulcers” are found. Usually these patients are afraid of doctors, are cancerphobic or manifest excessive denial may present late in the course of the disease with ghastly disfiguring facial destruction[1] .
2. Case Report
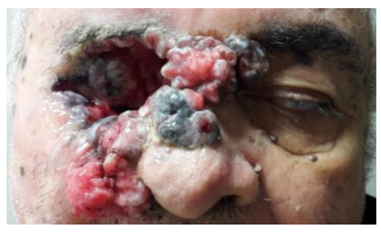
Patient refers periorbital tumor in the right side 7 years ago. Refers that it was initially a small tumor in the upper eyelid and it grew insidiously until involve the entire periorbital region and globe. Refers poor visual acuity in the left eye 8 months ago.
Visual acuity
Right eye (OD): no light perception.
Left eye (OS): count fingers 20 cm.
Inspection: extensive tumor on the right side, not visualized the globe. (photo1)
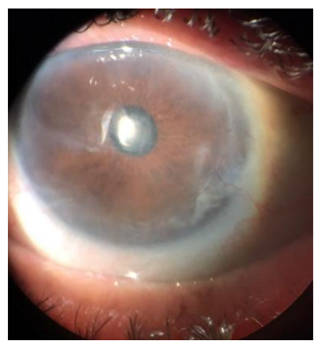
Biomicroscopy OS: symblepharo between superior tarsal conjunctiva and superior cornea, diffuse corneal opacities, nuclear cataract 2+/4+.
Fundoscopy OS: apparently applied retina, difficult to evaluate details due to opacities.
Patient and family members opted for no tumor resection because the left eye would be included in the surgical resection.
The patient had the desire to improve his left eye visual acuity.
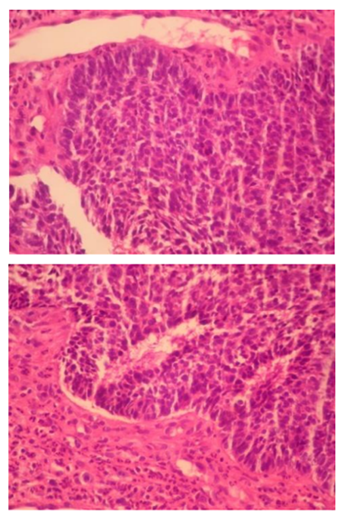
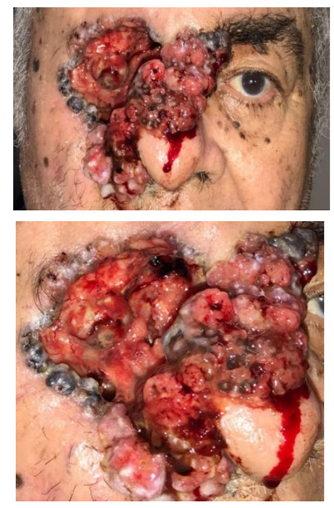
Initially it was performed the resection of the symblefaro and a biopsy of the lesion (photos 2 and 3). After, the cataract surgery with intraocular lens implantation was performed (photo 4).
In the postoperative the patient's visual acuity was OS 0.80 LogMAR, Jaeger 4. The patient was satisfied, he reported that he returned to do his daily activities, such as, bathing by himself, eating without any help,watching television, with great improvement in his quality of life (photos 5 and 6).
3. Discussion
Basal Cell Carcinoma (BCC) can be considered the most common malignant tumor. According to the American Cancer Society, more than 2 million people were treated in 2006 for non-melanoma skin cancer (NMSC), and most of the cases received the diagnosis of BCC. Some population studies estimate annual incidence for BCC in both Caucasian men and women to be approximately 146 cases per 100,000 persons[2] .
The exactly role of genetic in development of BCCs remains unclear. However, there are great quantities of evidences of genetic alterations in the development of BCCs, already described in many studies.
The TP53 gene is the most commonly mutated tumor suppressor in cancer. The mutations of this gene occur in, at least, 50% of cases[3] .
Sun exposure is the main environmental cause of BCC. A variety of studies has corroborated that intermittent, intense sun exposure appears to increase risk, but the cumulative effects of a long period of UV exposure does not.
Besides sun exposure, other risk factors are: ionizing radiation in the form of radiotherapy, the consumption of arsenic-contaminated water and arsenic-containing medications and immunosuppression in organ transplant recipients. There is no significant relationship between low CD4 count, indicating a lack of association between BCCs and immunodeficiency in HIV-positive people. Certain genetic syndromes are associated with the development of BCC, such as the nevoid basal cell carcinoma syndrome, in which patients can develop hundreds of BCCs and a variety of developmental abnormalities[4] .
The main clinical subtypes of BCCs are nodular, superficial, and morpheaform. Associations of superficial and morpheaform with nodular BCC may occur. Occasionally, variable amounts of melanin may be present within these tumors, which are often referred to as pigmented BCCs[5] .
Most cases of BCC appear on the face. The rest of the cases arise on the trunk and extremities generally.
Nodular BCC is the most common clinical subtype, (50 to 79% of all BCCs). Lesions consist of papules or nodules with a pearly,shiny quality and small arborizing telangiectasia. The tumor may enlarge and crusting may appear over a central depression[6] .
Superficial BCC is the second most common clinical subtype (15%). A lesion typically appears as a well-circumscribed, scaly, pink-to-red macule, patch, thin papule, or thin plaque. It may demonstrate crust or a thin rolled border consisting of fine translucent small papules. Areas of spontaneous regression can occur, leaving behind atrophic, hypopigmented areas[7] .
Morpheaform or sclerosing BCC corresponds for a low proportion of cases (5-10%). Its clinical resemblance to an indurated plaque of morphea, or localized scleroderma. Lesions present as pink-to-ivory-white, shiny, smooth, scar-like, indurated plaques or depressions with ill-defined borders and atrophy. Morpheaform BCC is usually more aggressive than the other types and it tends to exhibit a potential for extensive local destruction. It might be confused with scar, localized scleroderma, dermatofibrosarcoma protuberans, Merkel cell carcinoma, amelanotic melanoma, microcystic adnexal carcinoma, and other adnexal neoplasms[8] .
About the histopathological diagnosis, all subtypes of BCC present aggregations of basaloid keratinocytes, which are surrounded by stromal tissue and typically demonstrate a connection to the epidermis. Basaloid cells resemble the basal keratinocytes of normal epidermis and are characterized by intensely basophilic, large, relatively uniform nuclei, and scant cytoplasm. Tumor aggregates may demonstrate peripheral palisading of nuclei, and apoptotic cells are frequently[9] .
The aim of treatment of BCC is to completely remove the tumor, and maximally preserve either function or esthetic at the site of treatment. The selection of treatment depends, partly, on the risk of malignancy recurrence. Radiation therapy has been reported to result in low recurrence rates for both primary BCC (7.4%) and recurrent BCC (9.5%). It is often the primary option for the treatment of BCCs if surgery is contraindicated for some reason[10] .
Summering it, Basal Cell Carcinoma is the most common malignancy, and, although distant metastasis is not frequent, it may lead to extensive local destruction. The sun exposure is known the main environmental risk factor, and the certain role of genetic alterations needs additional researches. The bases of correct treatment are the full remove the tumor, besides the maintenance of function or esthetic at the site of tumor. In some circumstances, the curative treatment is not able to be performed. Therefore, efforts in attempt to improve the quality of life of these patients become our main goal.
References
- Eagle, Ralph C. Eye Pathology: An Atlas and Text, 2nd Edition. Copyright ©2010 Lippincott Williams & Wilkins Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C. et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998; 391(6662):90–92.
- O’Toole EA, Ponten F, Lundeberg J, Asplund A. In: Dermatology. 3rd edition. Bolognia JL, Jorizzo JL, Schaffer JV, editors. Saunders; 2012. Principles of Tumor Biology and Pathogenesis of BCCs and SCCs; pp. 1759– 1772.
- Youssef KK, Van Keymeulen A, Lapouge G, Beck B, Michaux C, Achouri Y. et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol. 2010; 12(3):299–30.
- Chuang TY, Popescu A, Su WP, Chute CG. Basal cell carcinoma. A population-based incidence study in Rochester, Minnesota. J Am Acad Dermatol. 1990; 22(3):413–417.
- Gorlin RJ, Goltz RW. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med. 1960; 262:908–912.
- Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A. et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996; 85(6):841–851.
- Hutchin ME, Kariapper MS, Grachtchouk M, Wang A, Wei L, Cummings D. Et al. Sustained Hedgehog signaling is required for basal cell carcinomaproliferationandsurvival: conditional skin tumorigenesis recapitulates the hair growth cycle. Genes Dev. 2005; 19(2):214–223.
- McCormack CJ, Kelly JW, Dorevitch AP. Differences in age and body site distribution of the histological subtypes of basal cell carcinoma. A possible indicator of differing causes. Arch Dermatol. 1997; 133(5):593–596.
- Gallagher RP, Hill GB, Bajdik CD, Fincham S, Coldman AJ, McLean DI. et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer. I. Basal cell carcinoma. Arch Dermatol. 1995; 131(2):157– 163.