Information
Journal Policies
Does Nutrition Underlay the Pathogenesis of Glaucoma and Cataract in Ageing?
Thekkuttuparambil Ananthanarayanan Ajith*
Copyright : © 2017 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
World Health Organization has been reported glaucoma, cataract, diabetic retinopathy, age- related macular degeneration, and trachoma as major causes for visual impairment. Among them, cataract remains the major avoidable blindness. The risk for cataract gradually increases in elder adults. More than 82% of people above the age of 50 years are under the risk for visual impairment [1]. Nutrition is one of the important determinants of health is often under diagnosed over the age of 65 [2]. Though no uniformly accepted definition for malnutrition has been described in the elder people, certain indicators had already been described for a specific vitamin deficiency [3]. During the last decade several studies had suggested the nutritional deficiency of certain vitamins and supplementation of iron as the modifiable risk factor for visual morbidity in the elder people.
Glaucoma is a chronic and irreversible optic neuropathy, due to the progressive loss of retinal ganglion cells (RGC). Multiple factors had been described for the incidence of glaucoma. Despite the etiological factors, the main goal of the current modalities of treatment is to alleviate the intraocular pressure (IOP), one of the confirmed modifiable risk factors. Some subjects continue to show substantial progression of the disease even with a significant lowering of IOP. Therefore, identification of modifiable risk factors other than IOP may provide additional therapeutic targets in such subjects. Senile cataract occurs after the age of 45 is also a multifactorial disease. Cataract is most common in South India and a number of indicators of poor nutrition have been found to be associated with its increased risk.
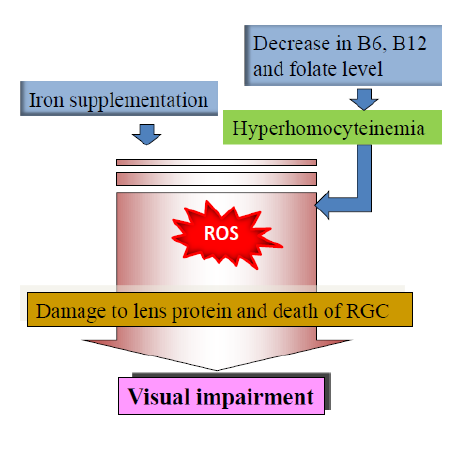
Recent findings suggested a close association of nutrition in cataractogenesis and glaucoma formation during aging. Among them, supplementation of iron and deficiency of certain vitamins remains the major factors. Accumulation of iron has been reported in a variety of tissues and has been increasingly implicated in the pathogenesis of aging and disease [4]. A chronic oxidative stress (OS) alters the intracellular iron homeostasis in human trabecular meshwork (TM) cells, resulting in the accumulation of redox-active iron mainly within the lysosomal compartment [5].This is occurring during the supplementation of iron > 18 mg/day. Study demonstrated that such supplementation was associated with significantly greater odds of self-reported glaucoma [6]. An exponential decrease in the cellularity of TM during aging was found in glaucoma donors [6]. Study in primary cultures of TM cells demonstrated that this may probably due to an increase in ferritin level during aging and, thereby, the iron content. Elevated iron content in the angle region has also been described in ceruloplasmin/hephaestin double- knockout mice [7].
Another nutrition related factor is hyperhomocysteinemia (HHcy) which is the elevated level of non-protein derived sulphur containing amino acid homocysteine (Hcy) in blood. The role of HHcy as causative agent in ocular diseases was recently reviewed as well [8]. HHcy is a condition occurring due to hereditary or non-hereditary defect in the metabolism of Hcy. Non-hereditary defect can be correlated to the status of B complex vitamins such as pyridoxine (B6), folic acid and cobalamine (B12) which are involved in the conversion of Hcy back to cysteine and methionine. Studies demonstrated that plasma total Hcy concentration is inversely related to the intake and plasma level of folate, B6 and B12 [9]. Therefore, the plasma concentration of total Hcy may indicate the nutritional status of these B-vitamins [10]. Many previous prospective studies reported significant high level of Hcy in glaucoma patients particularly in pseudoexfoliative glaucoma (PEXG) [11, 12]. A decreased serum folic acid level was found in PEXG group, while both folate and B12 were decreased in cataract patients [12, 13]. Though a decreased serum folic acid level was found in PEXG group, no statistically different serum B12 and folate levels reported in patients with other type of glaucoma [14]. This indicates that HHcy as one of the risk factors for other forms of glaucoma is debatable. Many studies reported the lower level of B12 and folate with an increase in age of elders irrespective of gender which may be explained with multiple etiological factors including intestinal atrophy. Such deficiency can finally result in HHcy.
The exact molecular basis of age-related iron accumulation remains elusive. Differences in expression of iron-regulating genes and their protein levels were found between the RGC of glaucomatous and nonglaucomatous eyes [15]. Iron may play important roles in glaucoma pathogenesis by impacting aqueous humor outflow and/or RGC survival. The accumulated iron favours the Fenton’s reaction to enhance the production of free radical and, thereby, the OS. Enhanced OS can initiate an irreversible damage to single layer epithelial cells of the lens and also damage the protein, crystallin. This can lead to necrotic or apoptotic cell death and lens opacification. A recent study has shown that TM cells have the highest oxygen consumption and is the most sensitive tissue to oxidative radicals in the anterior chamber of the eye [16]. Furthermore, OS can induce the apoptosis of RGC which has been observed in glaucoma. Despite the OS and apoptosis, the major mechanisms of iron in glaucoma formation during ageing remain elusive.
The molecular mechanisms underlying the ocular diseases in HHcy has been reported as impaired vascular endothelial function, apoptosis of RGC, extracellular matrix alterations, decreased lysyl oxidase activity and OS. The toxic effect of HHcy was also mediated through the direct cytotoxicity and pro- inflammatory properties of the Hcy derived homocysteine-thiolactone which can induce lens opacification and optic nerve damage. HHcy has role in glaucoma formation which can be correlated to apoptosis of RGC that may impair the optic nerve head blood supply [17].
Hence, a novel therapeutic strategy is inevitable to counteract the effect of OS in aging to prevent the visual morbidity. Despite the mixed results of association of HHcy and glaucoma, the levels of Hcy, folate, B6 and B12 should be measured early in patients with visual impairment and may supplement with these vitamins in order to attenuate the ocular damages. The role of iron in glaucoma and cataract was demonstrated experimentally but need to be evaluated in epidemiologic studies.
References
- http://www.who.int/mediacentre/factsheets/fs28 2/en/ (Updated August 2014)]
- Chandra RK. Nutrition and the immune system from birth to old age. Eur J Clin Nutr. 2002; 56(Suppl 3):S73–76.
- GuigozY, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The mini nutritional assessment, Clin. Geriatr. Med. 2002; 18: 737–757.
- Kurz T, Terman A, Gustafsson B, Brunk UT. Lysosomes in iron metabolism, ageing and apoptosis. Histochem Cell Biol. 2008; 129:389–406.
- Izzotti A, Sacca` SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003; 114:638–646.
- Wang SY, Singh K. Lin SC. The association between glaucoma prevalence and supplementation with the oxidants calcium and iron. Invest Ophthalmol Vis Sci. 2012; 53:725- 731.
- Hadziahmetovic M, Dentchev T, Song Y, Haddad N, He X, Hahn P, Pratico D, Wen R, Harris ZL, Lambris JD, Beard J, Dunaief JL. Ceruloplasmin/hephaestin knockout mice model morphologic and molecular features of AMD. Invest Ophthalmol Vis Sci. 2008; 49:2728–2736.
- Ajith TA, Ranimenon. Homocysteine in ocular diseases. Clin Chim Acta. 2015; 450:316-321.
- Sen SK, Pukazhvanthen P, Abraham R. Plasma homocysteine, folate and vitamin B12 levels in senile cataract. Find out how to access preview- only content Indian Journal of Clin. Biochem. 2008; 23:255-257.
- Turgut B, Kaya M, Arslan S, Demir T, Güler M, Kaya MK. Levels of circulating homocysteine, vitamin B6, vitamin B12, and folate in different types of open-angle glaucoma Clin. Inter. Aging 2010:5 133–139.
- Wang G, Medeiros FA, Barshop BA, Weinreb RN. Total plasma homocysteine and primary open-angle glaucoma. Am J Ophthalmol 2004, 137:401- 406.
- Leibovitch I, Kurtz S, Shemes G, Goldstein M, Sela BA, Lazar M. Hyperhomocysteinemia in pseudoexfoliative glaucoma. J Glaucoma. 2003, 12:36-39.
- Glaser TS, Doss LE, Shih G, Nigam D, Sperduto RD, Ferris FL, Agrón E, Clemons TE, Chew EY. The Association of Dietary Lutein plus Zeaxanthin and B Vitamins with Cataracts in the Age-Related Eye Disease Study: AREDS Report No. 37. Ophthalmol. 2015; 122:1471-1479.
- Turgut B, Kaya M, Arslan S, Demir T, Güler M, Kaya MK. Levels of circulating homocysteine, vitamin B6, vitamin B12, and folate in different types of open-angle glaucoma. Clin Interv Aging. 2010; 5:133-139.
- Gye HJ, Kim JM, Yoo C, Shim SH, Won YS, Sung KC, Lee MY, Park KH. Relationship between high serum ferritin level and glaucoma inSouth Korean population:the Kangbuk Samsung health study. Br J Ophthalmol 2016; 100:1703-1707.
- Lin Y, Epstein DL, Paloma B. Liton PB. Intralysosomal iron induces lysosomal membrane permeabilization and cathepsin D– mediated cell death in trabecular meshwork cells exposed to oxidative stress. Invest Ophthalmol Vis Sci. 2010; 51: 6483-6495.
- Wagenfeld L, Weiss S, Klemm M, Richard G, Zeitz O. Vascular dysfunction in ocular blood flow regulation: impact of reactive oxygen species in an experimental setup. Invest Ophthalmol Vis Sci. 2014;55:5531-5536