Information
Journal Policies
Protective Role of Sodium Selenite on the Toxic Effect of Lead Nitrate on Human Erythrocytes
Dilek Pandir1*,Fatih Oguz Bekdemir2,Ali Demirbag1
2.Bozok University, Graduate School of Natural and Applied Sciences, Department of Biology, 66100 Divanliyolu/Yozgat, Turkey.
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
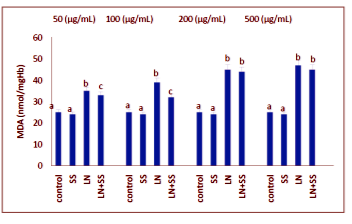
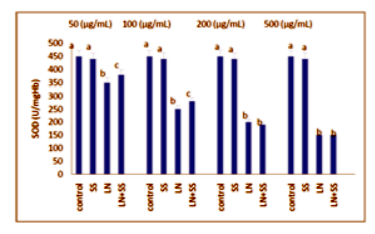
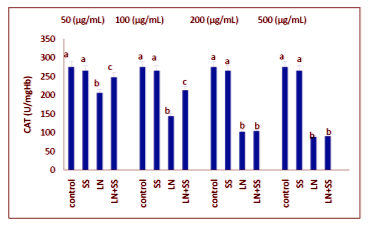
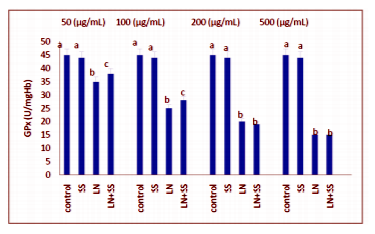
This work showed that the effects of the industry using lead nitrate (LN), which is widely used, and sodium selenite (SS) from antioxidants on the levels of malondialdehyde (MDA) and superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) activities in human erythrocytes in vitro. Erythrocytes was incubated under various treatment conditions (lead nitrate and/or sodium selenite) at 37°C for 60 min and the levels of MDA, SOD, CAT and GPx activities, were determined. CAT activity was measured according to the method of Aebi. GPx activity was measured according to the method of Paglia and Valentin. SOD activity was measured according to the method of Marklund and Marklund. MDA level was measured according to the method of Ohkawa. The experimental groups were analyzed by the software program SPSS 11.0 for Windows. Treatment with LN alone increased the levels of MDA and decreased SOD, CAT and GPx activities in erythrocytes (P < 0.05). SS-pretreated erythrocytes showed a significant protection aganist the cytotoxic effects induced by LN on the studied parameters as significant statistically.
LN, SS, toxicity, antioxidant enzymes, erythrocytes, human,Nutrition and Growth
1. Introduction
One of the most important pollutants for nature is heavy metals and more than 40 metals and alloys are used in the industry. Pollution brought by the heavy metals into the water is reaching to human health a threatening level [1]. Heavy metals have a relatively high density compared to other metals and they are toxic or poisonous even at low concentrations [2] on the metabolic functions of living organisms after certain levels and even cause their deaths [3].
Some of the inorganic salts such as lead acetate, lead nitrate (Pb(N03)2) are soluble in water. There is plenty of lead as an element in nature and transported to the seas and oceans from natural resources in various ways [4]. After the lead is taken in the body, it is transferred to the blood circulation and distributed to the soft tissues and the bone [5].
Selenium is an antioxidant trace element for animal and human tissues. This element has two inorganic forms as selenite and selenate [6]. Sodium selenite has anticarcinogenic and antimutagenic effects. Recent studies have shown the protective effect of selenium on oxidative damage caused by heavy metals [7]. Sodium selenite has higher tumor inhibition properties more than the organic forms [8]. Selenium prevents malignant transformation of normal cell [9]. The anticarcinogenic effect of selenium probably depends on the inhibition of MDA formation [10].
Erythrocytes are disc-shaped blood cells and contain less water than other tissue cells [11]. Erythrocytes play a role in the transport of substances such as hemoglobin, organic and inorganic phosphate with ions such as O2, CO2, Ca2+, Mg2+, Na+, K+, CO32-, Cl- [12]. The lead disrupts sodium-potassium adenosine triphosphatase (Na+/ K+ -ATPase) pump by interacting with phospholipids in erythrocyte membranes [13]. Thus reducing the level of normal phospholipid in the membrane and causing damage to the membrane. Eventually erythrocytes are hemolyzed [14].
Lead is one of the major agent that cause environmental pollution. In the past century, lead has started to accumulate in environment because of widespread use in industry. For this reason humans and animals were exposed to the lead [15]. It is known that lead is toxic to the nervous, cardiovascular, respiratory, digestive and endocrine systems [16-18]. Lead is also known to decrease the level of antioxidants, increase free radicals and lipid peroxidation [19]. As lead increases oxidative stress and lipid peroxidation, the DNA damage is inevitable and this is the main cause of lead-induced tissue damage [20].
However, currently there is no obtainable information considering the protective effects of sodium selenite (SS) against several different doses of lead nitrate (LN)-induced toxicity in the human erythrocytes. To do this, we analyzed the potency of SS against LN on the levels of MDA and antioxidative stress parameters such as SOD, CAT and GPx activities in blood tissue.
2. Material And Methods
At increasing doses of LN was used and this substance was supplied from Merck. SS and all other chemicals used in the experiments was obtained from Sigma-Aldrich.
For this study, 20 ml blood sample was taken from heparinized tubes in healthy 6 males who do not use any non-alcohol, and are not exposed to any chemicals in their working environment. Heparinized whole blood was centrifuged at 3000 rpm for 15 minutes. Plasma and leucocytes are removed and the erythrocytes are washed three times with physiological saline solution (0.9% NaCl), then 50% (v/v) cell suspensions with the same solution was prepared with PBS.
Erythrocytes were divided into two groups: control group (n = 6) and treatment group (n = 18). The treatment group is divided into three groups. These;
Group 1: LN treated group (n = 6),
Group 2: SS treated group (n = 6),
Group 3: LN + SS treated group (n = 6).
The chemicals were added to the erythrocytes and incubated for 1 hour at 37 °C. Erythrocytes that are waiting at -20 °C until working hours was diluted 4 times with cold deionized water to obtain hemolysis. Hemolysate samples results compared with the control group of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) enzyme activities from antioxidant defense enzymes system and malondialdehyde (MDA) level.
MDA forms a pink-colored complex resulting in an incubation at 90 °C with thiobarbituric acid (TBA) under aerobic conditions. The absorbance of this complex is read at 532 nm wavelength in the spectrophotometer. Analysis and analytical calculations was done according to Ohkawa et al., (1979). Place each test tube on the homogenate from TBA prepared in trichloroacetic acid (TCA). After mixing with the vortex, the tube is capped and left in the water bath for a while. Tubes from the water bath are centrifuged at 4000 rpm for 10 minutes to obtain supernatant and the absorbance is read in the spectrophotometer at 532 nm against the blind tube. The amount of malondialdehyde (MDA) will be given in nmol / mg Hb.
The total SOD was determined by the Marklund and Marklund [21] method and the absorbance of pyrogallol was measured by autoxidation at 440 nm in alkaline medium. When this enzyme activity was measured, the enzyme source was added by placing the Tris-EDTA buffer in plastic baths and the supernatant in varying volumes. By adding pyrogallol to these mixtures, autooxidation of pyrogallol was initiated. The total SOD activity per mg of protein in homogenate was then given as U/ mg Hb.
The activity of catalase enzyme was determined by the method specified by Aebi [22]. Add Triton X-100 (v/v) to remove the catalase from the supernatant peroxisomes obtained before reading absorbance in the spectrophotometer, then dilute by adding phosphate buffer. We have use the spectrophotometer (in the UV wavelength), place the glass sphere on the last diluted sample, add hydrogen peroxide and initiate the enzymatic reaction. Measured at 240 nm. The enzyme activity is given in U/mgHb.
Glutathione peroxidase was assayed according to the method specified by Paglia and Valentine [23]. This method was based on the measurement of the reduced absorbance of the glutathione reductase using oxidase glutathione (GSSG) and NADPH as the substrate at 340 nm due to the oxidation of nicotinamide adenine nucleotide hydrogen phosphate (NADPH). As the oxidant glutathione was formed by glutathione peroxidase, the reduction of NADPH is directly proportional to GPx activity. Oxidation of NADPH to nicotinamide-adenine-dinucleotide phosphate (NADP) leads to a decrease in absorbance at 340 nm, thus indirectly used in the detection of the activity of GPx. To measure the specific activity of this enzyme, Tris-HCl buffer, reduced glutathione, diluted supernatant, NADPH, glutathione reductase were added to the wells and incubated at room temperature. Hydrogen peroxide was added to this mixture to initiate the enzymatic reaction and the absorbance decreases at 340 nm. The specific activity of the enzyme was given in U/mgHb.
Differences between the groups was compared and statistically examined through the SPSS program. The statistical data obtained from the different dose groups was evaluated using the One Way Variance Analysis (ANOVA) and Tukey test in the Windows SPSS 11.0 computer program. A value of P <0.05 was be considered statistically significant.
3. Results
When the SS, LN, and LN plus SS treated groups were compared with the control group at the end of one hour, there were significantly increased in the MDA levels of the blood tissues. The MDA levels were decreased statistically significantly in the SS plus LN treated group compared to LN treated group (P < 0.05, Figure.1).
Compared to the control group, there were statistically significantly decreased in the SOD, CAT and GPx activities in the LN treated groups at the end of the one hour. However, SOD, CAT and GPx activities were increased in LN and SS treated groups compared with the LN treated group in blood tissue (P < 0.05, Figures 2-4).
4. Discussion
The heavy metals are noted to be as severe pollutants in environment due to their toxicity and stable structure [24,25]. Nowadays heavy metal emissions into the environment have considerably increased because of the industrial activity [26,27]. Thereby increasing the contamination and accumulation of heavy metals in the food chain [28].
Many studies have been carried out on the effect of LN on living organisms. Kürkçü et al.(2001) [29] have identified a 96-hour of LC50 value of LN which is used in industrial activities as a toxic contaminant in the aquatic ecosystem on Lepistes (Poecilia reticulata) fish and also found behavioral changes in individuals against different concentrations of LN. Sitemoğlu et al. gave mercuric chloride (0.02 mg / kg day), lead nitrate (45 mg / kg day) and lead nitrate + mercury chloride (45 mg/kg day lead nitrate+ 0.02 mg/kg day mercury chloride) to male rats by gavage and 4 weeks after treatment, histopathological changes in the small intestine in rats were investigated by light microscope and compared with the control group. Diseases caused by heavy metals are increasing day by day. In this study, we investigated that the effect of increasing doses of LN, one of the heavy metals, on human erythrocytes. The harmful effect of increasing concentrations of LN was also increased on antioxidative enzymes activities and MDA level.
Selenium is a structural part of some enzymes like glutathione peroxidases and thioredoxin reductase [30,31]. Selenium has detoxification effects on heavy metals [32]. Selenium is hopeful because of its antiradical activities so it could be used for antioxidant source for diet [33]. Yılmaz et al. [34] gave sodium selenite, mercury chloride, vitamin E + mercury chloride and sodium selenite + vitamin E + mercury chloride, sodium selenite, vitamin E, vitamin E + sodium selenite, mercury chloride, sodium selenite + mercury chloride to male rats by gavage during 4 weeks, histopathological changes in the thyroid tissue in rats were investigated by light microscope and compared with the control group. At the end of the study, they demonstrated that LN causes tissue damage in treatment groups and the SS can change this toxicity in rats. In another study, Adıgüzel et al.[35] investigated the effect of LN on the small intestinal tissue of diabetic and non-diabetic rats and the protective role of SS, which is known to have antioxidant properties. They showed that SS has not preventive effect on LN-induced toxicity in diabetic rats. In our study, it was found that SS was protective against low concentrations of LN but it did not show the same effect in the high doses of LN in erythrocytes of human.
Erythrocytes are one of the most important components that constitute between 40% and 45% of total blood volume. The erythrocyte membrane structure consists of three important components in order of, the phospholipid bilayer, transmembrane proteins and an underlying triangular spectrin protein network or cytoskeleton that is attached to the cytoplasmic side of the bilayer through short actin filaments or junction complexes [36]. In a study performed by Lovelock, hemolysis in human erythrocytes was associated with electrolyte concentration within the cell [37]. The use of erythrocytes in toxic studies is both easy and fast in terms of laboratory facilities. Erythrocyte membranes are highly sensitive to toxic substances and are very important in determining MDA levels. Antioxidative enzyme activities are active in erythrocytes and gives rapid reaction to toxic materials. For this reason erythrocytes from human blood cells were used in this study for determining MDA level and antioxidative enzyme activities.
Some enzymes of the cell are protective against cell damaging substances such as catalase (CAT), superoxide dismutase (SOD) and glutation peroxidase (GPx) [38]. Reactive oxygen species (ROS) leading to oxidative stress in erythrocytes in vitro through the generation of free radicals and being this damage in the defense system can lead to cell death [39].
The protective effects of SS on the human erthyrocytes antioxidative have not previously been described. Our findings indicate that LN (50, 100, 200 and 500 µg/mL) administration effect on erythrocytes depends on the concentrations. Different antioxidative enzymes activities and level of MDA were seen in the cell due to the sensitivity of the cells to LN after increasing exposure concentrations. 1.10-6 µM of SS has shown more impact on the cells at 50 and 100 µg/mL of LN when compared with 200 and 500 µg/mL of LN results according to studied parameters. However, further investigation is necessary to clarify the protective effect of SS suplementation on erythrocytes from cytological to molecular level.
Acknowledgements
The authors would like to thank to Kemal KOÇ and Fatma İLÇE for helping us to prepare this study.
References
- Güley M. Vural N., 1987. Toksikoloji, Ankara Üniversitesi Eczacılık Fakültesi Yayın. No: 48,(1987).
- Kahvecioglu Ö., Kartal G., Güven A., Timur S., TMMOB Metalürji Mühendisleri Odası, Metalürji Dergisi, 136, (2006).
- Uslu O., Türkman A., Su kirliligi ve Kontrolü, T.C Basbakanlık Çevre Genel Müdürlügü Egitim Dizisi. 1, 276-280 (1987).
- Kürkçü Y., Kursun Nitrat (Pb(NO3)2) Metal Tuzunun Lepistes (Poecilia reticulata) Üzerindeki Akut Toksik Etkisinin Araştırılması ve Davranış Değişimlerinin İncelenmesi, Gazi Üniversitesi Fen Bilimleri Enstitüsü, Eylül, (2001).
- Sonçağ A., Yurdakök K., İntrauterin toksik ağır metal etkilenimi, Çocuk Sağlığı ve Hastalıkları Dergisi. 53, 145-158 (2010).
- Luo Y., Zhang B., Cheng W., Wang Q., Preparation, characterization and evaluation of selenite-loaded chitosan/TPP nanoparticles with or without zein coating, Carbohydrate Polymers. 82, 942-951 (2010).
- Kalender S., Uzun F.G., Demir F., Uzunhisarcıklı M., Aslanturk A., Mercuric chloride-induced testicular toxicity in rats and the protective role of sodium selenite and vitamin E, Food Chem. Toxicol. 55, 456-462 (2013).
- Üstdal K.M., Karaca L., Türköz Y., Testereci H., Kuş S., Paşaoğlu H., Biyokimya. Malatya. Medipres. 170, 1 (2003).
- Dervis E., Oral antioksidanlar, Dermatoz, 2 (1), 263-267 (2011).
- Vural N., Toksikoloji, Ankara Üniversitesi Eczacılık Fakültesi Yayınları. 504-512, 541-552, 555-560 (2005).
- Ersoy E., Bayşu N., Biyokimya, Ankara Üniversitesi Veteriner Fakültesi Yayınları. No:408. Ankara. Yay. No:408388-429 (1986).
- Gözükara E.M., Biyokimya 2. Baskı, Evin Matbaası, Malatya. 258-271 (1994).
- Şanlı C., Hızel S., Albayrak M., (2005). Kurşun ve Çocuk Sağlığı, Sürekli Tıp Eğitimi Dergisi. 14 (4), 70-75 (2005).
- Patrick L., Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity, Altern. Med. Rev. 11, 114-127 (2006).
- Landrigan P.J., Boffetta P., Apostoli P., The reproductive toxicity and carcinogenicity of lead: a critical review, Am. J. Ind. Med. 38, 231-43 (2000).
- Bizarro P., Acevedo S., Nin˜o-Cabrera G., Mussali-Galante P., Pasos F., Avila-Costa M.R., Ultrastructural modifications in the mitochondrion of Mouse Sertoli cells after inhalation of lead, cadmium or lead-cadmium mixture, Reprod.Toxicol. 17(5), 561-6 (2003).
- Goswami K., Gachhui R., Bandopadhyay A., Hepatorenal dysfunctions in lead pollution, J. Environ. Sci. Eng. 47(1), 75-80 (2005).
- Mudipalli A., Lead hepatotoxicity & potential health effects, Indian. J. Med. Res. 126(6), 518-27 (2007).
- Yu D.Y., Li W.F., Deng B., Mao X.F., Effects of lead on hepatic antioxidant status and transcription of superoxide dismutase gene in pigs, Biol. Trace. Elem. Res. 126(1–3), 121– 128 (2008).
- Xu J., Lian L.J., Wu C., Wang X.F., Fu W.Y., Xu L.H.i Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice, Food. Chem. Toxicol. 46(5), 1488–1494 (2008).
- Marklund S., Marklund G., Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase, Eur. J. Biochem. 47, 469-474 (1974).
- Aebi H., Catalase in vitro. Methods. Enzymol, 105, 121-126 (1984).
- Paglia D.E., Valentine W.N., Studies on the quantative and qualitative characterization of glutathione peroxidase, J. Lab. Med. 70, 158-165 (1987).
- Tezcan Ö., Pandır D., Baş H.,. The effects of cadmium on enzymatic antioxidant system and lipid peroxidation of human erythrocytes in vitro and the protective role of plasma level of vitamins C and E. Pol. J. Environ. Stud. 21(6),1849-1854 (2012).
- Krishna A.K., Mohan K.R., Distribution, correlation, ecological and health risk assessment of heavy metal contamination in surface soils around an industrial area, Hyderabad, India. Environ. Earth Sci. 75, 411-427 (2016).
- Nicholson F.A., Smith S.R., Alloway B.J., Carlton-Smith C., Chambers B.J., An inventory of heavy metals inputs to agricultural soils in England and Wales, Sci. Total Environ. 311, 205-219 (2003).
- Satarug S., Baker J.R., Urbenjapol S., Haswell-Elkins M., Reilly P.E., Williams D.J., Moore M.R., A global perspective on Cd pollution and toxicity in non-occupationally exposed population, Toxicol. Lett. 137, 65–83 (2003).
- Nriagu J.O., Pacyna J.M., Quantitative assessment of worldwide contamination of air, water and soils by trace metals, Nature. 333, 134–139 (1988).
- Kürkçü Y., Gül A., Kurşun nitrat (Pb(NO3)2) metal tuzunun Lepistes (Poecilia reticulata) üzerindeki akut toksik etkisinin araştırılması ve davranış değişimlerinin incelenmesi, Yüksek Lisans Tezi, Gazi Üniversitesi Fen Bilimleri Enstitüsü, (2001).
- Su L., Wang M., Yin S.T., Wang H.L., Chen L., Sun L.G., Ruan D.Y., The interaction of selenium and mercury in the accumulations and oxidative stress of rat tissues, Ecotoxicol. Environ. Safe. 70, 483-489 (2008).
- Agarwal R., Behari J.R., Role of selenium in mercury intoxication in mice, Ind. Health. 45, 388-395 (2007).
- Diplock A.T., Watkins W.J., Heurson M., Selenium and heavy metals, Ann. Clin. Res. 18, 55-60 (1986).
- Amara I.B., Soudani N., Hakim A., Troudi A., Zeghal K.M., Boudawara T., Zeghal N., Selenium and vitamin E, natural antioxidants, protect rat cerebral cortex against dimethoate-induced neurotoxicity, Pestic. Biochem. Phys. 101, 165-174 (2011).
- Yılmaz F.M., Kalender Y., Civa klorid'in ratlarda tiroit bezi dokusu üzerine subakut toksisitesi ve sodyum selenit'in ve vitamin e'nin koruyucu rolü, Yüksek Lisans Tezi, Gazi Üniversitesi Fen Bilimleri Enstitüsü. (2014).
- Adıgüzel Ç., Kalender Y., Lead nitrate induced toxic effects on small intestine tissues in diabetic and non-diabetic rats: role of sodium selenite, GU J. Sci. 28(4), 541-544 (2015).
- Byers T.J., Branton D., Visualization of the protein associations in the erythrocyte membrane skeleton, Proc. Natl. Acad. Sci. USA. 82, 6153-6157 (1985).
- Lovelock J.E., The haemolysis of human red blood-cells by freezing and thawing, Biochim. Biophys. Acta. 10, 414–426 (1953).
- Faix S., Faixová Z, Michnová E.,Várady J., Effects of per os administration of mercuric chloride on peroxidation processes in Japanese Quail. Acta Vet. Brno 72: 23-26. (2003).
- Durak D., Kalender S., Uzun F.G., Demir F., Kalender Y., Mercury chloride-induced oxidative stress in human erythrocytes and the effect of vitamins C and E in vitro, African J. Biotechnology. 9(4), 488-495 (2010).