Information
Journal Policies
Stereotactic Radio Surgery for Residual and Recurrent Central Neurocytoma
Faye Mohameth1*, Diallo Moussa2, Rakotovao Ketsia1, Elhadji Cheikh Ndiaye SY2, Borius Pi�rre Yves1, Regis Jean Marie1
2.Service de Neurochirurgie, Hopital Nord, chemin des Bourrely 13915, Marseille, France.
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract:
Background: Central neurocytoma (CN) is a rare benign tumor of central nervous system that is typically located in the lateral ventricle and constitute 0.1-0.5% of all primary brain tumours.
Methods: We are relating retrospectively the results of 11 patients who underwent Gamma k nife radio surgery for CNs in the Gamma unit at functional and stereotactic neurosurgery service in Marseille from January 1999 to December 2015.The mean age at Gamma k nife radio surgery (GKS) was 25.12 years (range17-41 years). All patients underwent surgery before (GKS) with a mean delay of 19, 37 months (6 - 62months). The prescribed mean dose delivered to the tumour margin was 19, 1 Gy(12-24Gy).The mean volume of treatment was 4.4mL (1.03-10.7mL). The mean time follow-up was 55.2 months (7.2-108 months).
Results: The local tumor control was 90% at the final follow -up time. One recurring tumour was local (4.67years after GKS) and 2 were "out-of-field" recurrence (5.42 and 6.8 years after GKS) .Repeat stereotactic radio surgery was performed in two patients with an average delay between the two of 5.91years and an average dose of 14.34Gy. The local tumour control was also unrelated to the prescribed dose (p= 0.89) and the tumour volume (p= 0.67). No deaths and no radiation injury were observed during our follow-up.
Conclusion: Gamma k nife radio surgery is now the treatment of choice for sub-totally resected and local central neurocytoma recurrence with higher tumour control rates and low complications despite the small number of patients studied in the literature.
Central neurocytoma - Gamma k nife surgery- stereotactic radiosurgery.
CN: Central neurocytoma, MRI: Magnetic Resonance imaging, GKS: Gamma k nife surgery, SRS: Stereotactic radio surgery, RT: Radiation Therapy, VPS: Ventriculo -peritoneal shunt, CSF: Cerebro- spinal fluid, ARE: Adverse radiation effect.
1. Introduction
Central neurocytoma (CN) is a rare brain tumor. They are typically located in the lateral ventricle near the foramen of Monro [1]. And are classified as World Health Organization (WHO) grade 2. These tumours were first described as a distinct tumour entity by Hassoun et al. in 1982 [2]. CN constitute 0.1-0.5% of all primary brain tumours [3-6]. They are typically found in adolescents and young adults; with a higher incidence in the third decade, followed by the second and the fourth decades [7-9]. The male-to-female ratio is 1 [9-12].
They are typically intraventricular and can occur hydrocephalus. Surgical removal is the standard treatment but, complete removal of the lesion is often difficult because of the deep location [13]. A combination of treatments, such as surgery with adjuvant radiation, can be considered for these lesions [14,15]. A few number of studies have been published. Some authors have concluded that radiotherapy (RT) or stereotactic radio surgery can successfully control any residual tumour after incomplete resection or recurrence [16,-21].
The surrounding structures are therefore exposed to limited amounts of radiation. High-dose radiation for local lesions is possible with radio surgery. There are few reports on the efficacy of GKS for treatment of CNs. These reports are limited by the small number of patients studied [22-25]. So, our study focuses on the efficiency of Gamma Knife radio surgery (GKS) in CN's treatment.
2. Materials and Methods
We are relating the results of 11 patients who underwent GKS for CNs in the Gamma unit at functional and stereotactic neurosurgery service in Marseille from January 1999 to December 2015. One patient was excluded because the follow-up data was unavailable. The study included 4men and 6 women. Their mean age at GKS was 25.12 years (range17-41 years). The initial symptoms were headache in 10 patients, hemiparesis in 2 cases, gait disturbance in 3cases, consciousness disturbance in 2 cases and ocular symptoms were found in 3 patients. All patients underwent surgery before GKS with a mean delay of 19, 37 months (6-62months). The resection was total in 2 patients, subtotal in 7 patients and one patient underwent only a biopsy. The histologic diagnosis was CN in all patients, and no atypical findings were noted. Seven (7) patients had a dilatation of ventricles. A ventriculo-peritoneal shunt was performed for hydrocephalus in only one patient.
The procedure for GKS was as follows: a Leksell G stereotactic frame was attached to the patient's head under local anesthesia, and a stereotactic magnetic resonance imaging (MRI) was performed. The treatments were planned with the Leksell gamma plan by the neurosurgeons. After confirming the tumour margin, an isodose curve fit to the tumour margin was made using 4 sizes of collimators. Finally, the prescribed mean dose delivered to the tumour margin was 19, 1 Gy(12-24Gy) with an isodose of 50%. The mean volume of treatment was 4.4cm3 (1.03- 10.7cm3). Treatments were carried out according to the dose plan of the Leksell gamma knife model B, model C, model AC and Icon.
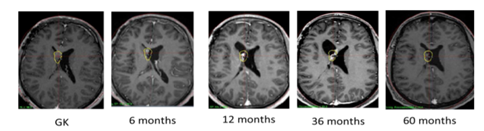
The follow up consists of a serie of cerebral MRI done at 6months post GKS, then, once a year for 5 years and the 7th and 10th years post- GKS. The procedure was successful when there where a stabilisation of the lesion, a diminution of the tumour size or the involution of the tumour on the follow up MRI done at 3 years post GKS or 5 years post-GKS.
The decision of GKS treatment was made by a multidisciplinary team which include: neuroradiologists, radiation therapists and neurosurgeons.
All factors that may affect the obliteration of the malformation, bleeding after the procedure and the occurrence of complications have been analysed with the statistical software SPSS 10 of Windows. Parameters with p < 0.05 were statically significant.
3. Results
On our study, 1 patient was lost from sight after the first control at 6 months, so we do not have his follow-up details. Of the remaining 10 patients, the mean time was 55.2 months (7.2- 108 months). No deaths were observed during our follow-up.
We had a volume reduction of all lesions treated, with an average of 86% (54-100%). Tumors were controlled locally in 7 cases including 3 cases who had a complete response. 3 patients had a partial response, and 1 case showed no change. The 3 and 5 year tumour's control were 100%, (Figure 1). One recurring tumour was local (4.67years after GKS) and 2 were "out-of-field" recurrence (5.42 and 6.8 years after GKS), A second treatment was performed in two patients with an average delay between the two of 5.91years. An average dose of 14.34Gy was performed and we had tumour control at 3 years of follow-up after the second GKS. In our follow-up, we had total lesional control in 9 patients (90%) during the whole follow-up period. The patient with local recurrence is still under surveillance.
During our follow-up, there were no deaths in our series. The radio surgical treatment stabilized the lesions in nine patients. No patients underwent a second surgery. A VP shunt was performed for obstructive hydrocephalus at 12 months of radio surgery treatment. The treatment resulted in the disappearance of symptoms in almost all patients except for the two patients who had an hemiplegia, probably linked to surgical trauma. The Karnofsky Performance Scale Score remain stable after treatment.
We did not find in our study an intra-lesional haemorrhagic complication as reported in the literature. But we had symptomatic side effects of the radiation; a case of hydrocephalus by foramen Monro obstruction which required a VP shunt at 12 months of treatment. Even if it cannot be completely attributed to the rays; because even the tumour can obstruct the channels of the CSF.
4. Discussion
CNs represent approximately 0.1-0.5% of all primary brain tumours [5,6]. Hassoun et al. described the first cases, since then, more than 500 cases have been described. These tumours typically affect adolescents and young adults with the highest incidence in the third decade, followed by the second and fourth decades [7-9]; it corroborates our study with a mean age of 25,12years (17-41years). The CN has been found in the cerebral hemispheres, spinal cord, brainstem, thalamus, amygdala, pineal gland, retina and cerebellum [2-8, 27, 28].
The treatment of choice for CN is complete resection of the tumour, usually leading to cure and long-term survival. However, in more than a half cases, the lesion cannot be completely resected [14, 15]. Gamma knife radio surgery is the treatment of choice for sub-totally resected and local CN recurrence [24, 25]. It is safer compared to conventional radiotherapy which can produce cognitive dysfunction and secondary tumour formation [20]. Although not statistically significant, Patel et al. [11] reported that adjuvant GKS for patients with STRs dem- onstrated 100% tumour control rate compared to patients without adjuvant fractionating radiation therapy (87% tumour control rate) [23]. Garcia et al. [20] also reported a higher tumour control rate of 93% with GKS versus 88% with fractionated radiation therapy.
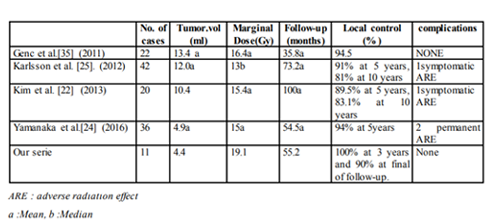
Schild et al. [23] related the first cohort of GKS for CNs, the local control rate of CNs after radio surgery is reported to be 91% and 100% with 15Gy of marginal dose. Cobery et al. [29] rewired 4 patients with sub-totally resected or recurrent CN treated with GKS with a follow-up periods ranging from 12 to 99 months. They found an important reduction in tumour size, between 48% and 81%. Bertalanffy et al [30]. Anderson et al.[31] and Tyler-Kabara et al.[32] also reported similar results with a series of 3 to 4 residual or recurrent CNs, they found that patients had relatively excellent responses to GKS (reduction in tumour size, without complications). Karlsson et al. [25] reviewed 42 patients with CNs treated with GKS. The median prescribed dose was 13 Gy (range, 11- 25 Gy), and the median tumour volume was 8 mL. They founded that the local tumour control rate was unrelated to the prescribed dose ((≤ 13 vs > 13 Gy, p=0.95) and was respectively 91% and 81% at 5 and 10 years after GKS. The local tumor control rate was also unrelated to tumour volume (< 7.55 vs > 7.55) cm3, p = 0.83).
Kim et .al [33] reported in a serie of 13 patients treated by radio surgery in first intension had a volume reduction of 50% with no complications over a 6-96month follow-up period. Chen and al.[34] GKS to treat 14 large CNs with a mean volume of 19.6 mL and a mean prescribed dose of 12.1 Gy, 100% decreased in the 5years following GKS and a mean reduction rate of 69%.Yamanaka et al.[24] reported a median rate of tumour volume reduction of 74% (range, 15%e100%). In the same report, the median prescribed dose was 15 Gy (range, 10-20 Gy), and the cumulative 5-year local control rate was 94%. On the basis of previous reports describing high local tumor control rates using a mean or median prescribed dose of 10.5-13 Gy. CN can be controlled with a relatively low prescribed dose (See table1).
In our series,the median prescribed dose and tumour volume were respectively 19.1 Gy and 4.4 mL. The cumulative local control rate was 90% at the last follow-up MRI. Our local tumour control was also unrelated to the prescribed dose (p= 0.89) and the tumour volume (p= 0.67) like in several reports [23-25].
We reported one local recurrence and 2 distant or out-of-field recurrence tumour years after radio surgery treatment. The last had a second session of radio surgery treatment for a local control, three years after the first procedure. Yamanaka et al. [24] related, local recurrence in 2 cases (5.6%) after GKS. Karlsson et al. [25] reported 2 local recurrence (2.4 and 9.6 years after GKS) and 2 distant or out-of-field recurrences (1.6 and 4.8 years after GKS) .Chen et al. [35] rewiered no recurrence after GKS in 14 CNs. Rades et al. [13] reported 1 recurrence in their 21 CNs, and Genc et al. [35] reported 1 recurrent case in their 22 CNs treated with GKS. The rate of local tumour recurrence is very low after GKS. Yen et al. [18] founded 2 out-of-field recurrences that were difficult to detect even with a retrospective review of imaging at the time of GKS in their 7 treated patients. Kim et al. [22] reported 3 out-of-field recurrences in their 20 CNs that were treated with GKS. All out-of-field recurrences occurred in cases undergoing surgery before GKS. Karlsson et al.[25] reported that a low incidence of distant recurrences does not justify a higher risk for long-term complications following radiotherapy. Local and distant tumour recurrences can be favourably managed using additional radiation treatment Yamanaka et al. [24] reported a radiation injury in 1 case and a slight memory disturbance persisted after the extent of the injury decreased. In this case, the tumour volume was 14.2 mL and the prescribed dose was16Gy. Martin et al. [36] founded 1 radiation necrosis case among their 4 CNs treated with linear accelerator radio surgery.
The size of the tumour in this case was 23 mL, and the prescribed dose was 16 Gy. Chronic edema and radiation necrosis appeared 2 years after treatment, the patient's symptoms improved after a ventriculoperitoneal shunt procedure and corticosteroid treatment [36]. Yen et al. [37] related a case that required a ventriculo-peritoneal shunt procedure due to hydrocephalus caused by an intratumoral hemorrhage 4 months after GKS. Gallina et al. [38] reported haemorrhage in the fourth ventricle and confirmed the absence of atypical histology with low proliferative potential after surgery. Terakawa et al. [39] also reported a hemorrhagic CN and speculated that approximately 3% of CNs was hemorrhagic. Karlsson et al. [25] reported no complications after GKS of 42 patients. In our study one patient developed hydrocephalus 12 months after GKS and required a ventriculo-peritoneal shunt. Yamanaka et al [24] reported that the incidence is low, a large lesion treated with a relatively high dose may tend to develop adverse radiation effects and the limit of the radio surgical dose for the fornix is not well known, symptoms associated with fornix injury may seldom occur with prescribed doses < 15 Gy.
5. Conclusion
CN is a benign tumour of the CNS with an excellent prognosis. Surgery with gross complete resection is correlated with the best long-term survival rate and local tumour control. GKS is an effective treatment for residual and recurrent CNs after surgery because of it high rate of local tumour control and the low morbidity.
6.Acknowledgement
We thank Professor Regis for allowing us to do this work and all of the service. We also thank all the authors who have treated this subject before us.
References
- Zhang B, Luo B, Zhang Z, Sun G, Wen J: Central neurocytoma: A clinicopathological and neuroradiological study. Neuroradiology, 2004; 46:888-895.
- Hassoun J, Soylemezoglu F, Gambarelli D, Figarella-BrangerD,von Ammon K, Kleihues P: Central neurocytoma: A synopsis of clinical and histological features. Brain Pathol, 1993;3:297-306.
- Leenstra JL, Rodriguez FJ, Frechette CM, Giannini C, Stafford SL, Pollock BE, Schild SE, Scheithauer BW, Jenkins RB, Buckner JC, Brown PD: Central neurocytoma: Management recommendations based on a 35-year experience. Int J Radiat OncolBiol Phys , 2007; 67:1145-1154.
- Louis DN, Ohgaki H, Wiestler OD. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97-109.
- Coca S, Moreno M, Martos JA, Rodriguez J, Barcena A, Vaquero J. Neurocytoma of spinal cord. Acta Neuropathol 1994; 87:537-40.
- Kim DG, Chi JG, Park SH, et al. Intra ventricular neurocytoma: clinic opathological analysis of seven cases. J Neurosurg 1992; 76:759-65.
- Sharma MC, Deb P, Sharma S, Sarkar C. Neurocytoma: a comprehensive review. NeurosurgRev 2006;29:270-85; discussion 285.
- Figarella-Branger D, Soylemezoglu F, Kleihues P, Hassoun J. Central neurocytoma. In: Kleihues P, Cavenee WK. Pathology and genetics of tumors of the nervous system. Lyon: IARC Press; 2000.p.107-109.
- Eng DY, DeMonte F, Ginsberg L, Fuller GN,Jaeckie K. Craniospinal dissemination of centraneurocytoma. Report of two cases. J Neurosurg.1997; 86:547-552.
- Maiuri F, Spaziante R, De Caro ML, Cappabianca P, Giamundo A, Iaconetta G. Central neurocytoma: clinico-pathological study of 5 cases and review of the literature. ClinNeurol Neurosurg 1995; 97:219-28.
- Patel DM, Schmidt RF, Liu JK. Update on the diagnosis, pathogenesis, and treatment strategies for central neurocytoma. J Clin Neurosci 2013;20:1193-9.
- Vasiljevic A, Francois P, Loundou A, et al. Prognostic factors in central neurocytomas: a multicenter study of 71 cases. Am J SurgPathol 2012; 36:220-227.
- Rades D, Fehlauer F. Treatment options for central neurocytoma. Neurology 2002; 59:1268-70.
- Yang I, Ung N, Chung LK, et al. Clinical manifestations of central neurocytoma. NeurosurgClin N Am 2015; 26:5-10.
- Choudhari KA, Kaliaperumal C, Jain A, et al. Central neurocytoma: a multi-disciplinary review. Br J Neurosurg 2009;23:585-95.
- Rades D, Fehlauer F, Lamszus K, et al. Well- differentiated neurocytoma: what is the best available treatment? NeuroOncol 2005; 7:77-83.
- Rades D, Schild SE. Treatment recommendations for the various subgroups of neurocytomas. J Neurooncol 2006;77:305-9.
- Chen H, Zhou R, Liu J, Tang J. Central neurocytoma. J Clin Neurosci 2012;19:849-53.
- Seung J. Lee, Timothy T. Bui, Cheng Hao Jacky Chen, et al. Central Neurocytoma: A Review of Clinical Management and Histopathologic Features. Brain Tumor Res Treat 2016; 4(2):49-57.
- Garcia RM, Ivan ME, Oh T, Barani I, Parsa AT. Intraventricular neurocytomas: a systematic review of stereotactic radio surgery and fractionated conventional radiotherapy for residual or recurrent tumors. ClinNeurol Neurosurg 2014; 117:55-64.
- Chen YD, Li WB, Feng J, Qiu XG. Long-term outcomes of adjuvant radiotherapy after surgical resection of central neurocytoma. RadiatOncol 2014; 9:242.
- Kim JW, Kim DG, Chung HT, et al. Radiosurgery for central neurocytoma: long- term outcome and failure pattern. J Neurooncol 2013; 115:505-11.
- Schild SE, Scheithauer BW, Haddock MG, et al. Central neurocytomas. Cancer 1997; 79:790-5.
- Kazuhiro Yamanaka1, Yoshiyasu Iwai1, Takashi Shuto, Yoshihisa Kida et al. Treatment Results of Gamma Knife Radio surgery for Central Neurocytoma: Report of a Japanese Multi-Institutional Cooperative Study.J neurosurgery 2016; 90:300-305.
- Karlsson B, Guo WY, Kejia T, Dinesh N, Pan DH, Jokura H, et al. Gamma Knife surgery for central neurocytomas. Clinical article. J Neurosurg. 2012;117(suppl):96-101.
- Kane AJ, Sughrue ME, Rutkowski MJ, et al. The molecular pathology of central neurocytomas. J ClinNeurosci 2011; 18:1-6.
- Giangaspero F, Cenacchi G, Losi L, Cerasoli S, Bisceglia M, Burger PC. Extraventricular neoplasms with neurocytoma features. A clini- copathological study of 11 cases. Am J SurgPathol 1997; 21:206-12.
- Enam SA, Rosenblum ML, Ho KL. Neurocytoma in the cerebellum. Case report. J Neurosurg 1997; 87:100-2.10.
- Cobery ST, Noren G, Friehs GM, Chougule P, Zheng Z, Epstein MH, et al. Gamma knife surgeryfor treatment of central neurocytomas. Report of four cases. J Neurosurg. 2001; 94:327-330.
- Bertalanffy A, Roessler K, Koperek O, GelpiE, Prayer D, Knosp E. Recurrent central neurocytomas. Cancer. 2005; 104:135-142.
- Anderson RC, Elder JB, Parsa AT, Issacson SR, Sisti MB. Radio surgery for the treatment of recurrent central neurocytomas. Neurosurgery. 2001; 48:1231-1238.
- Tyler-Kabara E, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radio surgery for central neurocytoma. Report of four cases. J Neurosurg.2001; 95:879-882.
- Chae-Yong Kim, Sun Ha Paek, Sang Soon Jeong, Hyun-Tai Chung, et al.Gamma Knife Radio surgery for Central Neurocytoma Primary and Secondary Treatment Cancer. 2007; (110)10:2276-2284.
- Chen MC, Pan DHC, Chung WY, Liu KD, Yen YS,Chen MT, et al. Gamma knife radio surgery for central neurocytoma: retrospective analysis of fourteen cases with a median follow-up period of sixty-five months. Stereotact Funct Neurosurg. 2011; 89:185-193.
- Genc A, Bozkurt SU, Karabagli P, Seker A, BayriY,Konya D, et al. Gamma Knife radiosurgery for cranial neurocytomas. J Neurooncol. 2011;105:647-65.
- Martin JM, Katati M, Lopez E, Bullejos JA, Arregui G, Busquier H, et al. Linear accelerator radio surgery in treatment of central neurocytomas. ActaNeurochir (Wien). 2003; 145:749-754.
- Yen CP, Sheehan J, Patterson G, Steiner L.Gamma Knife surgery for neurocytoma. JNeurosurg. 2007; 107:7-12.
- Gallina P, Mouchaty H, Buccoliero AM, Lorenzo ND. Haemorrhagic central neurocytoma of the fourth ventricle. ActaNeurochir (Wien). 2005; 147:1193-1194.
- Terakawa Y, Tsuruno T, Ishibashi K, Okada Y, Shimotake K, Murata T. Central neurocytoma presenting with massive hemorrhage leading to coma. Case report. Neurol Med Chir (Tokyo). 2010; 50:139-143.