Information
Journal Policies
The Effect of Memantine on Prophylaxis of Chronic Migraine Headache
Davood Kashipazha1, Sahereh Emadi*1
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction: Migraine is a common headache disorder that is caused by increased central nervous system irritability and is one of the most debilitating diseases in the world. In recent years, there has been a lot of interest in the use of glutamate mediators for migraine prophylaxis, but there is still no research on the prophylaxis of chronic migraine headaches with memantine in our country. The purpose of this study was to investigate the effect of memantine on the improvement of the severity of chronic migraine headaches.
Methods: A total of 54 patients with chronic migraine referred to Golestan and Imam Hospital of Ahvaz were randomly divided into two groups: memantine and placebo. Both groups received 50 mg topiramate with a dose of 25 mg and doubled dose in two weeks. In the memantine group, memantine was administered at a dose of 20 mg daily, which reached to this dose 4 weeks. The severity, duration and frequency of migraine attacks at the beginning of the study, the end of the first month, the second month, and the third and fourth months of the study were recorded during the follow up.
Results: The mean pain intensity based on the VAS score was not significantly different between the two groups at the beginning of the study and at the end of the first month. At the end of the second month, however, the end of the third month and the end of the fourth month was significantly lower in the memantine group. At the beginning of the study, at the end of the first month and the end of the second month, the average number of days the person had migraine did not differ significantly between the two groups. However, at the end of the third month and the end of the fourth month, the mean of the days of migraine patients was significantly lower in the memantine group than in the placebo group. After 4 months of treatment, MIDAS score in the memantine group was 22.44 ± 10.62, while in the placebo group it was 14.74 ± 1.78 (p <0.0001).
Conclusion: According to the results of this study, there was a significant difference between the two memantine and placebo groups in decreasing the severity, frequency and duration of chronic migraine headaches after at least two months of treatment with memantine, indicating the effect of memantine on treatment and prevention Chronic migraine headaches.
1. Introduction
Migraine headache is a very common neurobiological headache disorder that is caused by increased central nervous system irritability and is among the most debilitating diseases in the world. Chronic migraine refers to a migraine headache in which the patient has had a migraine headache for more than 15 days per month for a period of at least 8 months, including at least 8 days of full migraine headache.
Most cases of chronic migraine are associated with a gradual increase in the frequency of migraine headaches [1]. Several extensive studies in Europe and the United States have shown that the prevalence of migraine headaches in women is about 20% and in men is about 6% [2]. According to estimates, 15 percent of the world's population is infected with migraine headaches. Migraine is a recurring and recurrent headache that has many effects on the patient, family, and community, due to its chronicity and high prevalence, and it imposes a heavy burden on various communities and social life each year [3,4]. Depression, chronic fatigue, maternity leave, family problems and drug dependence, are some of the psychosocial problems of chronic headaches. Even the symptoms are an inadequate picture of the effects of this disorder [5]. Research in this area suggests that migraine headaches, especially the chronic type, greatly affect the quality of life of patients. Migraines greatly limit the social activities of patients and reduce the ability to do home work and physical and non-physical activities of patients, and disturbs leisure [6,7].
Different methods such as medication and psychotherapy have been used to reduce headaches. All medications, in addition to their high levels of efficacy, have multiple side effects in long-term use [8,9]. In recent years, there has been a strong desire to use glutamate mediators for prophylaxis of migraine headaches, since it has been proven that glutamate or its receptors are involved in numerous structures associated with pain. Glutamate levels in CSF have been increased in patients with chronic migraine headaches and this suggests high secretion levels in patients with chronic migraine headaches [10,11].
Memantine is an NMDA receptor antagonist that acts through the blocking of pain signals called the glutamate system, which plays a major role in the pathophysiology of chronic migraine headaches [12]. Memantine acts as a low voltage dependent non-competitive antagonist to the NDMA receptor and prevents excessive intake of calcium into the neurons, thereby inhibiting excessive neuronal activity in pain relief pathways [13]. Therefore, this drug can be an appropriate and effective option for prophylaxis and the treatment of chronic pain, including chronic migraine headaches [14]. Several clinical studies have reported very uncommon side effects of memantine. Despite the research carried out in this area, there is still no significant and persuasive research on the prophylaxis of chronic migraine headaches with memantine in our country. The aim of this study was to investigate the effect of memantine on the prophylaxis of chronic migraine headaches.
2. METHODS AND MATERIALS
This study were designed as double-blind clinical trial. The research was approved by the Jundishapur University of Medical Sciences, IR.AJUMS.REC.1396.630 and licensed by the Ethics Committee of the University. A total of 60 patients with chronic migraine headaches referring to the neurology clinic of Golestan and Imam Hospitals of Ahvaz were enrolled. All of these patients were classified as chronic migraine headaches according to the International Headache Society (IHS).
MOH patients with barbiturates and opium; MOH patients with a history of abortions; excessive over-retention; and the need for hospitalization for bridge therapy, pregnancy or lactation, impaired renal or hepatic function, or cardiovascular disease, any psychiatric disorder results in non-obstruction of the protocol such as depression, bipolar disorder, history of memantine and topiramate susceptibility, history of glaucoma, kidney stones, kidney failure, severe hepatic failure, cardiovascular disease, hypersensitivity, or complications. Attributable to memantine and topiramate of these, 6 patients did not complete the study, and 54 patients remained till the end of the study. After explaining the research goals for patients, written consent was obtained and patients were randomly divided into two groups of memantine and placebo. In the memantine group, memantine was given at a dose of 20 mg per day, which reached this level within 4 weeks. Also, in both groups, with regard to the presence of chronic migraine headaches, 50 mg of topiramate was given as a slow-acting basal drug with a dose of 25 mg and an increase in the dose was doubled in two weeks. Severity of pain and the rate of disability and frequency of attacks at the beginning of the study, the end of the first month, the second month, the third month, and the fourth month of the study were recorded during the follow up.
MIDAS (Migraine Disability Assessment Test) questionnaire consists of 7 questions. The first to fifth questions relate to the rate of loss of function caused by migraine headaches. All headache questions have been answered within the past three months and the responses are based on the number of days. The total of these 5 questions based on the following breakdown will indicate the level of performance of the individual:
- 0 to 5, MIDAS Grade I, Little or no disability
- 6 to 10, MIDAS Grade II, Mild disability
- 11 to 20, MIDAS Grade III, Moderate disability
- 21+, MIDAS Grade IV, Severe disability
The data were entered into the statistical package of Statistical Software (SPSS) and analyzed. To compare the quantitative variables between each of the two groups, the Mann-Whitney test was used. Also, for the comparison of the mean of changes, repeated measurement was used. P <0.05 showed a significant difference.
3. RESULTS
From 54 patients, 27 patients were in the memantine group and 27 patients were in the placebo group. The mean age of patients in the memantine group was 37.99 ± 9.86 years and 39.67 ± 5.78 years in the placebo group (p = 0.004). In the memantine group, two patients were male and 25 were female, while in the placebo group, there were five males and 22 patients in the placebo group. The two groups did not have a significant difference in gender (p = 0.786).
Headache in the memantine group was unilateral in 10 patients and was bilateral in 17 patients. Also in the placebo group, it was unilateral in 8 patients and in 19 patients was bilateral (p = 0.773).
As shown in Table 2, at the beginning of the study, the average number of days for migraine Family history of migraine headache was observed in 16 patients in the memantine group (59.3%) and 13 patients in the placebo group 48.1%) (p = 586).
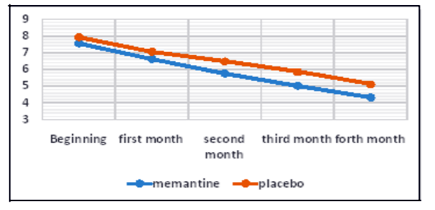
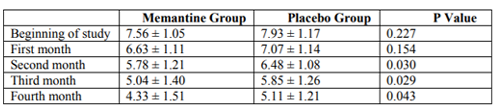
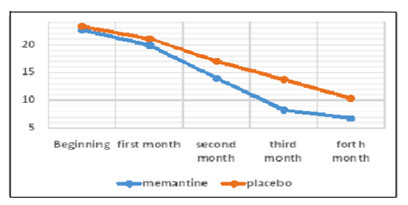
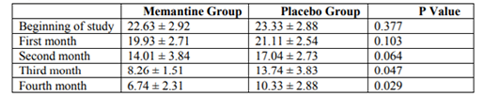
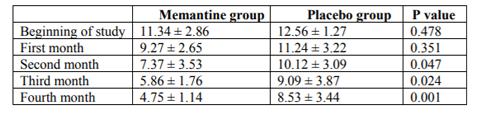
As shown in Table 1, the mean pain intensity based on the visual scale of pain measurement was 7.55 ± 1.05 in the memantine group and 7.93 ± 1.17 in the placebo group. The severity of pain was not significantly different in the two groups at the beginning of the study (p = 0.227). At the end of the first month, the mean pain intensity was 6.63 ± 1.11 in the memantine group and 7.7 ± 1.14 in the placebo group, which was not significantly different between the two groups (p = 0.154). At the end of the second month, the mean pain intensity was 5.78 ± 1.21 in the memantine group and 6.48 ± 1.08 in the placebo group (p = 0.30). At the end of the third month, the mean pain intensity in the memantine group was 5.4 ± 1.40 and in the placebo group was 5.85 ± 1.26 (p = 0.029). At the end of the fourth month, the mean pain severity in the memantine group was 4.33 ± 1.51 and 5.11 ± 1.21 in the placebo group (p = 0.043). Figure 1 shows the trend of change in pain severity over a four-month follow-up, comparing two study groups. Accordingly, the severity of pain was reduced in both groups during this period. However, these changes significantly decreased in the memantine group (p <0.0001) headache was 22.33 ± 2.92 days in the memantine group and 23.33 ± 2.88 days in the placebo group. At the beginning of the study, the number of days for migraine headache There was no significant difference between the two groups at the end of the first and second months (p = 0.103 and p = 0.178, respectively) in terms of the days when migraine headaches were present.
However, at the end of the third and fourth months, the number of migraine headache days was significantly lower in the memantine group than in the placebo group (p = 0.047, p = 0.029, respectively).
In Table 3, the mean number of daily-consumed doses in both memantine and placebo groups was compared over four months. There were no significant differences in the number of days in The MIDAS questionnaire was completed before and after the end of treatment (after 4 months) for all patients.
Before the beginning of the study, MIDAS score was 40.04 ± 13.66 in the memantine group and 39.37 ± 15.35 in the placebo group, which was not significantly different between the two groups (p = 0.864). At the end of the study, the MIDAS score in the memantine group was patients was not significantly different in two groups (p = 0.377).
Figure 2 shows the trend of changes in the incidence of migraine headaches over a four-month follow-up, comparing two groups. Accordingly, in both groups during this time, the frequency of days when the patient had migraine headaches decreased. However, these changes significantly decreased in the memantine group (p <0.0001) the two groups at the beginning of the study and the end of the first month, but at the end of the second, third and fourth months, there was a significant decrease in the memantine group.
17.59 ± 5.52 and in the placebo group was 24.63 ± 14.77, which was significantly lower in the memantine group (p = 0.024).
After 4 months of treatment, the MIDAS score in the memantine group was 22.44 ± 10.62, while in the placebo group it was 14.74 ± 1.78. In patients with memantine, the improvement of MIDAS score was significantly higher (p <0.0001).
4. DISCUSSION
The mean pain intensity based on the visual scale did not have a significant difference in the pain intensity at the beginning of the study and at the end of the first month between two groups. But at the end of the second month, the third month and the fourth month was significantly lower in the memantine group. In both groups, the severity of pain was reduced during the four months of treatment. But these changes were significantly reduced in the memantine group.
Also at the beginning of the study, at the end of the first month and the second month, the average number of days for migraine headache did not differ significantly between the two groups. However, at the end of the third month and the end of the fourth month, the mean of the days that patients had migraine headache was significantly less in the memantine group than in the placebo group. During both periods, the frequency of days when the patient had migraine headaches was reduced. But these changes were significantly reduced in the memantine group.
There was no significant difference in the duration of migraine headache between the two groups at baseline and one month after treatment. However, the duration of migraine headache in the memantine group at the end of the second, third and fourth months of treatment was significantly shorter than that of the placebo group.
After 4 months of treatment, the amount of migraine-headed bakeries decreased by MIDAS in the memantine group 22.44 ± 10.62 while in the placebo group it was 14.74 ± 1.78. In the memantine group, the improvement of MIDAS score was significantly higher.
Several studies have been done on the effects of memantine on the improvement of migraine headaches before our study, which is to compare the studies conducted with this study.
In a study by Bigal et al. on 28 migraine patients, patients were treated with memantine for three months. In this study, the average number of days with severe headache decreased from 7.8 to 3.2 days per month during the follow-up three months. Also, the average disability score was significantly reduced over the three months (from 54.9 to 36.6) [15].
As in our study, Charles et al. found that 36 out of 54 patients had a significant reduction in their headache frequency and improvement in their daily function during the 2 months after the start of treatment with memantine [7]. In the study of Krusz and Camarata, 20 patients with migraine headache were treated with memantine. It was shown that the frequency of migraine headaches decreased from 9.2 days per month to 1.4 days per month, and 56% reduction in the baseline frequency of migraine headache was seen in 14 of 20 patients [16].
In the Lindelof study, which was performed on 22 patients with chronic tension headache, after 10 weeks of treatment with memantine, the severity of headache in both sexes was reduced in patients who used memantine [17].
In a 2008 case report from a 75-year-old patient who had a history of migraine headache and migraine headache treated with memantine 10 mg daily, he found that after 5 months of using memantine, his quality of life improved. The patient's symptoms were completely resolved [18].
The concentration of glutamate in the blood and CSF in patients with migraine headaches increases. In addition, stimulant production increases glutamate levels in pain-related structures. Glutamate receptors have been identified in several pain-related structures, including Trigeminal ganglion, Trigonomoservical complex and Thalamus. Therefore, the relationship between glomerular and migraine systems should be considered [19]. Glutamate plays an important role in several pathways associated with migraine headaches. These pathways include central stimulation, trichomoniosecular response, and diffuse corticosteroid wave (CSD). In a study titled The Effect of Selective NMDA Receptor antagonists in the Spinal Fluoresistive Wave in Rats in 2007 by Peeters et al. In the UK, it was found that the NMDA subtype of NR2B receptor is a means for suppressing corticoplasty. For example, memantine and other NR2B selective antagonists may be useful as new therapies for migraine and other CSD-related disorders, such as stroke and brain damage. However, chronic use is more effective than acute use [20]. In addition, excessive glutamate has been shown to play an important role in the persistence of migraine headaches [21].
Memantine is able to compete with magnesium and prevents long-term calcium entry, thus preventing nerve stimulation, and therefore can be considered for migraine headache prophylaxis [22].
The most important benefit of memantine with other migraine prevention drugs is its side effects. Other medications for migraine headaches often have significant side effects. Hair loss and weight gain are unpleasant in using valproate, especially for women who are mainly migraine headaches. Topiramate can have side effects and paresthesia, while venlafaxine may be associated with digestive symptoms [23]. In addition, unlike many migraine medications, memantine is considered as a Group B drug during pregnancy, which is suitable to prevent migraine headaches in pregnant women. With these results, memantine has a clearer benefit than other prophylactic drugs for migraine headaches.
5. CONCLUSION
According to the results of this study, there was a significant difference between the two memantine and placebo groups in reducing the severity, frequency and duration of chronic migraine headaches after at least two months of treatment with memantine, which indicated the effect of memantine on the treatment and prevention of chronicmigraine headaches. Also, the incidence of migraine headache was significantly reduced in the memantine group compared to placebo at the end of the study.
References
- Schwedt TJ. Chronic migraine. BMJ (Clinical researched). 2014;348:g1416.
- Cooke LJ, Becker WJ. Migraine prevalence, treatment and impact: the canadian women and migraine study. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2010;37(5):580-7.
- Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia : an international journal of headache. 2010;30(9):1065-72.
- Lipton RB. Chronic migraine, classification, differential diagnosis, and epidemiology. Headache. 2011;51 Suppl 2:77-83.
- Buse D, Manack A, Serrano D, Reed M, Varon S, Turkel C, et al. Headache impact of chronic and episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache. 2012;52(1):3-17.
- Barbanti P, Fofi L, Cevoli S, Torelli P, Aurilia C, Egeo G, et al. Establishment of an Italian chronic migraine database: a multicenter pilot study. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2018.
- Charles A. Migraine. The New England journal of medicine. 2017;377(6):553-61.
- Jurgens TP. [Update migraine therapy]. MMW Fortschritte der Medizin. 2011;153(14):49-52.
- Tepper SJ, Spears RC. Acute treatment of migraine. Neurologic clinics. 2009;27(2):417-27.
- Hoffmann J, Charles A. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2018.
- Niddam DM, Lai KL, Tsai SY, Lin YR, Chen WT, Fuh JL, et al. Neurochemical changes in the medial wall of the brain in chronic migraine. Brain : a journal of neurology. 2018;141(2):377-90.
- Assarzadegan F, Sistanizad M. Tolerability and Efficacy of Memantine as Add on Therapy in Patients with Migraine. Iranian journal of pharmaceutical research : IJPR. 2017; 16(2):7 91-7.
- Tipton AF, Tarash I, McGuire B, Charles A, Pradhan AA. The effects of acute and preventive migraine therapies in a mouse model of chronic migraine. Cephalalgia : an international journal of headache. 2016; 36 (11) :1048-56.
- Noruzzadeh R, Modabbernia A, Aghamollaii V, Ghaffarpour M, Harirchian MH, Salahi S, et al. Memantine for Prophylactic Treatment of Migraine Without Aura: A Randomized Double-Blind Placebo-Controlled Study. Headache. 2016;56(1):95-103.
- Bigal M, Rapoport A, Sheftell F, Tepper D, Tepper S. Memantine in the preventive treatment of refractory migraine. Headache. 2008; 48(9):1337-42.
- Cammarata D, Krusz J. Memantine for migraine prophylaxis. J Pain. 2006; 7(4):S43.
- Lindelof K, Bendtsen L. Memantine for prophylaxis of chronic tension-type headache-- a double-blind, randomized, crossover clinical trial. Cephalalgia: an international journal of headache. 2009; 29(3):314-21.
- Spengos K, Theleritis C, Paparrigopoulos T. Memantine and NMDA antagonism for chronic migraine: a potentially novel therapeutic approach? Headache. 2008; 48(2):284-6.
- Chan K, MaassenVanDenBrink A. Glutamate receptor antagonists in the management of migraine. Drugs. 2014; 74(11):1165-76.
- Peeters M, Gunthorpe MJ, Strijbos PJ, Goldsmith P, Upton N, James MF. Effects of pan- and subtype-selective N-methyl-D-aspartate receptor antagonists on cortical spreading depression in the rat: therapeutic potential for migraine. J PharmacolExpTher. 2007; 321(2):564-72.
- Gorji A, Scheller D, Straub H, Tegtmeier F, Kohling R, Hohling JM, et al. Spreading depression in human neocortical slices. Brain Res. 2001; 906(1-2):74-83.
- Filatova E, Latysheva N, Kurenkov A. Evidence of persistent central sensitization in chronic headaches: a multi-method study. J Headache Pain. 2008; 9(5):295-300.
- Whyte CA, Tepper SJ. Adverse effects of medications commonly used in the treatment of migraine. Expert Rev Neurother. 2009; 9(9): 1379-91.