Information
Journal Policies
ARC Journal of Neuroscience
Volume-1 Issue-3, 2016, Page No: 10-16
Rationale for Curcumin Therapy in Alzheimer’s Disease
Panchanan Maiti1, 2, 3, 4*, Gary L. Dunbar1, 2, 3, 4
1.Field Neurosciences Institute laboratory for Restorative Neurology.
2.Program in Neuroscience.
3.Department of Psychology, Central Michigan University, Mt. Pleasant, MI.
4.Field Neurosciences Institute, St. Mary’s of Michigan, Saginaw, MI.
2.Program in Neuroscience.
3.Department of Psychology, Central Michigan University, Mt. Pleasant, MI.
4.Field Neurosciences Institute, St. Mary’s of Michigan, Saginaw, MI.
Citation : Agata VD, Maugeri G, Cognata VL, et al. Expression Profile of Human PARK2 Splicing Isoforms in Peripheral Blood Cells and Isolated Lymphomonocytes. ARC Journal of Neuroscience. 2016;1(3):1–9. doi:10.20431/2456-057X.0103001
Copyright : © 2016 Agata VD. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The key pathology of Alzheimer’s disease (AD) is the progressive accumulation of misfolded amyloid beta proteins (Aβ) in extracellular and intracellular spaces. It is an age related, complicated disorders involving multifactor, including neuro-inflammation, oxidative damage along with Aβ deposition. These events may work either independently, or together lead to neuronal degeneration, thus perturb neuronal communications, which ultimately show a long term cognitive and motor dysfunction. The therapy for this disease is still unclear and under active investigation. Although several synthesized compounds, small molecules, drugs have been investigated to prevent further neurodegeneration, some of them have demonstrated partial inhibition of aggregation of the Aβ and their neurotoxicity, but unfortunately, most of them show unsatisfactory outcome. However, recently, as a potent anti-amyloid and anti-inflammatory natural polyphenol curcumin (the principal ingredient from the root extract of Curcuma longa) has been used in several neurodegenerative diseases, including AD. It can reduce amyloid burden, rescue neuronal damage and restore normal cognitive and sensory motor functions in different animal models of AD. Interestingly, it is not only neuroprotective, but also has preferential binding to misfolded Aβ. Therefore, Cur has been considered a promising natural compound to use as therapeutics as well as detection of Aβ plaques in AD. This mini-review highlighted the therapeutic targets, including possible molecular pathways of curcumin action to rescue neurodegeneration in AD. This article should help to researcher for basic understanding of the molecular mechanism of action of curcumin to use as therapeutics to fight against neurodegeneration in AD.
Keywords: Neurodegenerative Diseases, Amyloidosis, Curcumin, Neuroinflammation, Anti-Amyloid
Abbreviations: AD-Alzheimer’s Disease, Aβ-β-amyloid peptide, NFT-neurofibrillary tangle, Cur-curcumin, EC-effective concentration, APP- Amyloid precursor protein, BACE- β-secretase, BBB-blood brain barrier, MAP-microtubule associated protein, PHF-paired helical filaments, GSK-3β-glycogen synthase kinase-3β, MAPK-mitogen-activated protein kinase, pTau-phosphorylated tau, DHA- docosahexaenoic acid, IRS-Insulin receptor substrate, JNK-Jun amino-terminal kinases, IL-interleukin, TNF-tumor necrosis factor, IFN- interferon, COX-cyclooxygenase, PET-positron emission tomography, NIR-near infrared, PiB-Pittsburgh compound B, FDG-Fludeoxyglucose
1.Introduction
Alzheimer’s disease is the major age related neurodegenerative disease characterized by early memory deficits, followed by gradual decline of cognitive and intellectual functions or dementia, along with neurobehavioral abnormalities (Brendan et al., 2007). It is one of the leading causes of death for elder population worldwide. The hallmark pathology of AD is the deposition of amyloid beta protein (Aβ) as senile plaques in extracellular spaces, and phosphorylated tau as neurofibrillary tangle (NFT) intracellularly (Masashi et al., 2012). The accumulation of these abnormal or misfolded proteins are thought to be the principal reason for synaptic deficits, neuronal loss, oxidative damage and increase neuroinflammation in numerous brain regions (Chen et al., 2012). Most importantly, the pre-clinical signs of AD, including accumulation of Aβ are present decades before its clinical onset and most of the late-stage of AD (Reisa et al., 2011). Therefore, prognosis is essential to start therapy in order to prevent or delay the progressive neurodegeneration in AD.
Although several research have been done to prevent or delay AD progression using anti-amyloid, anti-inlfamatory agents, small molecules, drugs, but none of them are in satisfactory level yet. However, as a potent anti-amyloid natural polyphenol, curcumin attracted to the researchers to use as a promising drug of choice for therapy of AD (Frautschy et al., 2000, Ono et al., 2004; Ma et al., 2013; Begum et al., 2008; Maiti et al., 2014). It can bind and inhibit the Aβ aggregation, and improve motor coordination and cognition in mouse models of (Yang et al., 2005; Ono et al., 2004; Begum et al., 2008; Maiti et al; 2016). Interestingly, it has strong affinity towards Aβ, thus can be utilized to detect early pathology in animal’s models of AD (Maiti et al., 2016). In addition, as a low cost, easily available natural fluorochrome and strong binding affinity to Aβ, recently curcumin has been used as a potent labeling probe for imaging Aβ plaques and help to detect AD in their early stage non- invasively (Garcia-Alloza, 2007; Ran et al., 2009; Maiti et al; 2016). Several analogues and derivatives of Cur have been formulated to increase its imaging capability and implemented for imaging Aβ-plaques in animals and human AD (Ran et al., 2009). Because of it’s poor water solubility, instability in body fluids, rapid degradation, and limited bioavailability, curcumin is less attractive for treatment of AD. Recently, several formulas of Cur have been developed, including liposome-curcumin, or Cur conjugated with nanogel or dendrimer or with silver, or gold nanoparticle and also with solid lipid nano particles. We have been using the solid lipid cur particles (Longvida), to increase its solubility, bioavailability, and which have been tested in vitro and in different animal models of AD (Ryu et al., 2006 ; Begum et al., 2008; Koronyo et al., 2012 ; Ma et al., 2013 ; Zghang et al., 2015; Maiti et al ; 2014 ; 2015 ; 2016). In this mini review, the potential roles of Cur in AD therapy are highlighted. In short, along with its therapeutic efficacy, curcumin can be used as a promising imaging fluorochrome for detection of Aβ-plaques and diagnosis of AD non-invasively.
2.Curcumin Source
The principal yellow pigment present in the turmeric root extract of Curcuma longa, (a ginger family, Zingiberaceae) is curcumin, which is a diarylheptanoid in nature. Biochemical analysis showed that curcuminoids complex found in whole turmeric contains about 2.5-6% pure curcumin (Lee et al., 2013). The Commercial curcumin contains many components including three main types of curcuminoids, such as (a) curcumin (diferuloylmethane/Curcumin-I, ∼77%), (b) demethoxy curcumin (Curcumin-II, ∼ 17%) and (c) bis-demethoxy curcumin (Curcumin-III, ∼ 3%). Besides that four identified turmerones (among them alpha-turmerone, beta-turmerone, ar-turmerone, and aromatic- turmerone), as well as alpha-santalene, aromatic-curcumene, curlone, and other compounds were found (Anand et al., 2007; 2008; Aggarwal et al., 2007).
3. Rationale For Curcumin Therapy In Neurological Diseases
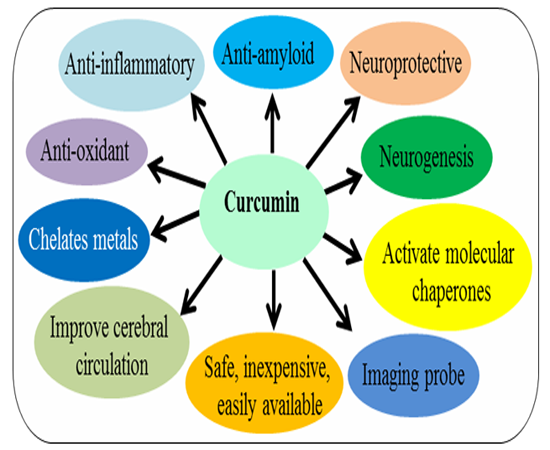
Several experimental data suggest that curcumin has pleiotropic effects on nervous system (Frautschy et al., 2001; Cole et al., 2004; 2007; Yang et al., 2005; Begum et al., 2008; Maiti et al., 2014; 2015; 2016). It is a neuroprotective agent, with potent antioxidant, along with anti-inflammatory activity (Cole, 2007). It’s anti-amyloid properties makes it most promising compounds for treatment of different brain diseases caused by amyloid accumulation (Yang et al., 2005; Lim et al., 2000; Mutsuga et al., 2012; Maiti et al., 2016). Several experimental data suggest that neuroinflammation, oxidative damage and deposition of misfolded amyloid proteins synergistically contribute the pathogenesis of many neurological diseases, therefore targeting these processes is to be relevant for these diseases therapy (Ono et al., 2004; Yang et al., 2005; Begum et al., 2008; Maiti et al., 2014; 2015). There are several reasons for Cur therapy in neurological diseases, such as: (i) it can easily cross blood brain barrier; (ii) it can bind and disaggregate amyloid oligomers and fibrils (anti-amyloid); (iii) it can enhance amyloid clearance similar to vaccine; (iv) it can reduce chronic inflammation in neurodegenerative diseases; (v) it is a potent anti-oxidant; (vi) it can stimulate neurogenesis (animal studies); (vii) It can chelate metals, remove metals from Aβ; (viii) there is no side effects even taking up to 12g/day; (ix) it is inexpensive and easily available; (x) it is chemically hydrophobic or lipophilic in nature which helps to increase its absorption in brain and increase its half-life; (xi) it can produce high fluorescent intensity when it binds to amyloid-plaques, therefore, can be used for labelling and imaging of amyloid plaques in-vitro and in-vivo, or can be used imaging probe for non-invasive techniques (Cole 2007; Begum et al., 2008; Maiti et al., 2016).
4. Curcumin In Alzheimer’s Disease
- Inhibition of Aβ aggregation: Numerous experimental data demonstrated that Cur can directly bind to β-pleated sheet structures of Aβ in-vitro (Ono et al., 2004; Yang et al., 2005; Maiti et al., 2016). Interestingly, the Cur showed the strongest inhibitory effect on Aβ aggregation among 214 antioxidant compounds tested in-vitro (Kim, H. et al. 2005), indicating it is one of the most potent anti-amyloid compounds investigated so-far. An in vitro study conducted by Ono et al. have demonstrated that Cur have a dose dependent effects on the inhibition of Aβ1−40/1−42 fibrils with an EC50 of 0.09–0.63 μM (Ono, K. et al. 2004). Several in vitro studies demonstrated that Cur can attenuate the assembly of both Aβ40 and Aβ42 oligomers and fibril formation Ono et al., 2004). Oral or intraperitoneal injection of Cur for 3-7 days to mice, cross blood brain barrier and found in brain tissue and decrease neuropathology in animal model of AD as shown by two-photon imaging (Zhang et al., 2014). Similarly, significant inhibition of Aβ oligomerization, its plaques formation, and decrease tau phosphorylation were observed along with behavioral improvements in an AD mouse models after oral intake of Cur (Lim, 2001; Cole, 2003; Yang, 2005). Further, in vivo imaging using multiphoton microscope showed a decrease of 30% Aβ plaque size and also prevent dystrophic neurites when the animals were injected the Cur via tail vein for one week (Garcia-Alloza, 2007). Another study conducted by Koronyo-Hamaoui et al., (2012) showed that Cur also binds with Aβ- plaques in retina. A clinical study showed that Cur engulf Aβ effectively and decrease plaque load in AD brain. Though there are no true epidemiological studies of Cur intake and the incidence of AD, but one surprising trend is observed among Indian and south Asian countries those who consume Cur everyday as a spice, have less development of AD compared to the United States and other Western countries (David et al., 2005).
- Inhibits Aβ production: Aβ is a by-product of a transmembrane protein called amyloid precursor protein (APP). The production of Aβ is catalyzed by the two successive enzymes, first by β-secretase (BACE) followed by γ-secretase, which contain presenilin-1 (PS1). It is speculated that during disease progression, induction of inflammatory signals aggravates the expression of Aβ production by increasing the activity of BACE. Whereas, Cur inhibits the activity of BASE, thus reduce level of Aβ (Frautschy et al., 2010; Lin et al., 2008). In addition, Cur is a potent inhibitor for APP metabolic pathway, thus lower the Aβ level (Zhang et al., 2010). Further, it can regulate Aβ production by inhibiting GSK-3β-mediated PS1 activation (Xiong et al., 2011).
- Aβ clearance: The levels of Aβ in the brains of AD depend on a balance between production, clearance and influx. The levels of Aβ are increased due to impairment of clearance pathways. However, there are several ways Aβ are disposed from the cell, including receptor-mediated Aβ transport across the blood–brain barrier and enzyme-mediated Aβ degradation and involvement of immune system for Aβ clearance. Research reports suggest that Cur can act as amyloid vaccine (by Frautaschy et al., 2010, Maiti et al., 2014). It can bind with Aβ and able to remove Aβ from the brain promoting receptor-mediated Aβ efflux from the brain. In contrast, curcumin could decrease Aβ load by suppressing the Aβ influx across the blood brain barrier (BBB), and upregulate the enzyme- mediated degradation of Aβ. Furthermore, Cur stimulates phagocytosis and increase the association of phagocytic cells around Aβ-plaques as observed in a rat AD model (Frautschy, 2001) and Tg2576 mouse model of AD, as well as with plaques in human sections exposed to primary rodent microglia (Cole, 2004).
- Inhibition of phosphorylated tau: AD pathogenesis is also involved aggregation of abnormal tau. Tau is a microtubule binding protein or microtubule associated protein (MAP), which is essential for microtubule stabilization. They are abundant in neurons of central nervous system. Alteration of tau protein structure causes damage of cytoarchitecture leading to oxidative stress, mitochondrial dysfunction and neurodegeneration (Kunner et al., 1998; Gamblin et al., 2000; Zhu et al., 2002). In AD, tau become hyperphosphorylated and form paired helical filaments (PHF) and deposited as neurofibrillary tangle (NFT) leading to neurodegeneration. Several tau kinases are involved for deposition of NFTs including glycogen synthase kinase-3β (GSK-3β) and mitogen-activated protein kinase (MAPK). However, similar to other amyloid proteins, Cur has been shown to bind to NFTs in human AD brain and mouse model of AD (Mohorko et al.,, 2010; Mutsuga et al., 2012). It inhibits pTau in-vitro by reducing oxidative stress. Similarly, previously we have reported that Cur can reduce Tau dimer and pTau oligomerization in a human tau transgenic mouse model (Ma et al., 2013), because Cur inhibits GSK-3β activity. Further, oral administration of Cur (555 ppm) together with DHA reduces pTau by inhibiting IRS-1 and JNK activities in vivo (Ma, 2009).
- Inhibits oxidation and inflammation: Though Aβ induced oxidative stress and neuroinflammation is not clear yet, but it is considered one of the primary events involved in neuronal death in AD (Garcia-Alloz et al., 2006; Behl et al., 1992; Mrakand et al., 2001). However, as a strong antioxidant, Cur can limit the pro-oxidant, pro-inflammatory and other toxic effects in AD brains. Cur can inhibit the inflammatory cytokines, including IL-1, IL-6, TNF-α, IFN-γ, and also COX-2 activity.
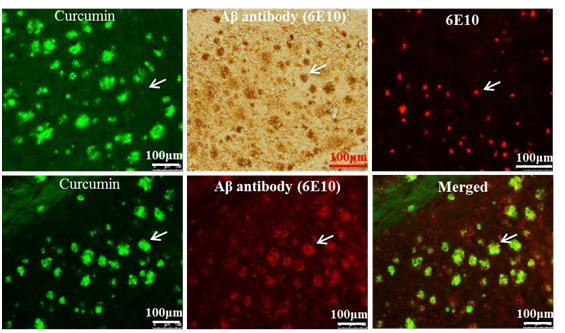
- As an imaging probe for Aβ-plaques detection ex-vivo and in vivo. Curcumin is naturally fluorescent and preferentially binds to Aβ plaques, therefore, it can be used to label and image Aβ- plaques ex-vivo and in-vivo (Maiti et al., 2016). In addition, it has structural similarities with classical amyloid binding dyes, (such as Thioflavi-s, Congo red, and crysamine-G), which makes it a promising candidate for labelling and imaging of amyloid plaques ex-vivo and in-vivo (Maiti et al., 2016). Garcia-Alloz et al. (2007) demonstrated that Cur can be used to visualize Aβ-plaques in vivo as shown in APP-tau transgenic mouse model. Similarly, a strong fluorescent signal was observed when the brain sections from animal models of AD and AD patients were incubated with Cur (Zhang et al., 2015). To confirm whether Cur binds to Aβ-plaques, the sections from mouse brain of AD was probed with Aβ-specific antibody (6E10) and then stained with Cur, and observed curcumin completely co-localized with Aβ-specific antibody, which indicates Cur has specificity to Aβ similar to Aβ-specific antibody (Figure 2) (Maiti et al., 2016)
More recently, using its fluorescent properties, researchers have tried to use Cur derivatives for in vivo imaging, such as potential positron emission tomographic (PET) probes for amyloid imaging or retinal scan for detection of AD in experimental animals and humans (Koronyo-Hamaoui et al., 2012). Whereas, it is not a practical probe for in vivo near infrared (NIR) imaging due to its short emission wave length (~550 nm), limited bioavailability, and rapid degradation. By modifying the structure of curcumin a boro-fluoro-Cur derivative have been developed which shift the emission wavelength to the near infrared (NIR) range. These derivatives are called CRANAD, and in this way they developed several derivatives including CRANAD-2, CRANAD-44 and CRANAD-28 (Ran et al., 2009). These derivatives of Cur probe significantly increases fluorescence properties upon binding to Aβ-plaques(Ran et al., 2009; Ryu et al., 2006). Surprisingly, the binding affinity of Cur for Aβ aggregates is higher (with a Ki of 0.07 nM for F18 labeled curcumin binding for fibrillar Aβ) than the molecular imaging probes, such as Pittsburgh compound B (PiB) in Fludeoxyglucose positron emission tomography (FDG-PET) (Ryu, 2006). Not only labelling Aβ-plaques, Cur can also help to visualize the distinct morphology of different Aβ-plaques, such as Core, neuritic, diffuse and burned out plaques, indicating that it can be used to investigate the overall amyloid plaque loads as well as helps to characterize the morphology of Aβ-plaques after anti-amyloid therapy (Maiti et al., 2016). Therefore, as a potent anti-amyloid polyphenol, Cur has a complete requisite profile for labelling and imaging the amyloid plaques (Maiti et al., 2016).
5. Recommended Doses And Limitations Of Curcumin Therapy
Toxicological evaluation revealed that Cur is found to be pharmacologically safe (up to 12g/day), or no apparent side effects has been observed in animal studies and in phase-I clinical trial (Agarwal et al., 2010). Similarly, another phase-1 human trial with 8g of Cur per day for three months found no toxicity (Agarwal et al., 2010). However, a few studies revealed that with high doses certain mild symptoms including gastro-intestinal upset, chest tightness, skin rashes, and swollen skin may be seen with some allergic reactions or dermatitis (Agarwal et al., 2010). Furthermore, chronic intake of Cur sometimes may be hepatotoxic. Therefore, the person having liver diseases, such as liver cirrhosis, biliary tract obstruction, gallstones, obstructive jaundice and acute biliary colic, or those are under prescribed medication for hepatic problems are not recommended for Cur therapy, because it can stimulate bile secretion. Similarly, alcoholics or heavy drinkers are not also recommended for this therapy. Furthermore, the individual taking any blood thinning agents, non-steroidal anti- inflammatory drugs or reserpine are not supposed to take Cur, because it can interacts with these drugs. In fact supplementation of even 20-40 mg of Cur per day can increase the gallbladder contractions in healthy people (Mishra et al., 2008). However, as we mentioned before, the dietary Cur is very unstable in most of the body fluids, and with poor water solubility and limited tissue bioavailability. Therefore, Cur mixed with oil would be better to absorb in our digestive system. Experimental animal results showed that 600 nM is sufficient to reduce pathology in mouse model of AD (Begum et al., 2008; Maiti et al., 2015). Extrapolation of animal studies to clinical trial revealed that an oral supplementation of Cur in the range of 80-500 mg /day are recommended to get its beneficial effect in human, whereas, intake of raw turmeric may be 2-4 gram/day (Mishra et al., 2008; Begum et al., 2008; Agarwal et al., 2010; Maiti et a., 2015).
6. Conclusion
Alzheimer’s disease is an age related complicated syndrome with a complex neuropathological characteristics, and develops progressively before showing any clinical symptoms. Neuronal damage and cognitive deficits or impairment of motor coordination are the major problems in this disease. Because of its pleotropic actions on nervous system, including anti-amyloid, anti-inflammatory and anti-oxidant properties as well as safe, inexpensive, easily available and effectively penetrates into the brain tissues, curcumin is a promising candidate for targeting protein misfolding neurological diseases, such as AD. It is also as effective as other fluorescent probe for imaging amyloid plaques in animal models as well as human AD brain. However, further research and clinical trials are necessary for its use in the therapy of AD.
7. Acknowledgement
Supports from the Field Neurosciences Institute, St. Mary’s of Michigan, is gratefully acknowledged.
References
- Masashi K., Rodrigo R., and Frank M.L.,. Transgenic Mouse Models of Alzheimer Disease: Developing a Better Model as a Tool for Therapeutic Interventions, Curr. Pharm. Des. 18(8), 1131–1147 (2012)
- Chen X., Guo C., and Kong J., Oxidative stress in neurodegenerative diseases Neural Regen Res.7(5), 376–385 (2012)
- Maiti P., Hall TC., Paladugu L., Koli N., Rossignol J., Dunbar DL., A comparative study of A comparative study of dietary curcumin, nanocurcumin, and other classical amyloid-binding dyes for labeling and imaging of amyloid plaques in brain tissue of 5×-familial Alzheimer's disease mice. Histochem. Cell Biol., 146(5), 609-625 (2016)
- Sperling RA., et al., Towards defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease Alzheimers Dement, 7(3): 280–292 (2011).
- Aggarwal, BB., Sundaram C., Malani N., Ichikawa H., Curcumin: the Indian solid gold, Adv. in Expt. Med and Biol, 595, 1-75 (2007)
- AggarwalBB., and Harikumar KB., Potential Therapeutic Effects of Curcumin, the Anti- inflammatory Agent, Against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases, Int. J. Biochem. Cell. Biol, 41(1):40-59 (2009)
- Anand P., Kunnumakkara AB., Newman RA., et al. Bioavailability of curcumin: problems and promises. Mol. Pharm. 4, 807-818 (2007)
- Cole GM., Teter B., and Frautschy SA., Neuroprotective effects of curcumin, Adv. Exp. Med.Biol, 595,197–212 (2007)
- Zhang X., Tian Y., Zhang C., et al., Near-infrared fluorescence molecular imaging of amyloid beta species and monitoring therapy in animal models of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America, 112, 9734-9739 (2015)
- Koronyo Y., Salumbides BC., Black KL., et al. Alzheimer's disease in the retina: imaging retinal abeta plaques for early diagnosis and therapy assessment, Neurodegener. Dis. 10, 285-293 (2012)
- Ryu EK., Choe YS., Lee KH., et al. Curcumin and dehydrozingerone derivatives: synthesis, radiolabeling, and evaluation for beta-amyloid plaque imaging. J. Med. Chem. 49, 6111-6119 (2006)
- Ran C., Xu X., Raymond SB., et al. Design, synthesis, and testing of difluoroboron-derivatized curcumins as near-infrared probes for in vivo detection of amyloid-beta deposits. J. Am. Chem. Soc. 131, 15257-15261(2009)
- Zhang X., Tian Y., Yuan P., et al. A bifunctional curcumin analogue for two-photon imaging and inhibiting crosslinking of amyloid beta in Alzheimer's disease. Chemical communications, 50, 11550-11553 (2014)
- David DC., Hauptmann S., Scherping I., Schuessel K., Keil U., Rizzu P., Ravid R., Drose S., Brandt U., Muller WE., Eckert A., Gotz J., Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J. Biol. Chem. 280(23), 802–23, 814 (2005)
- Ono K., Hasegawa K., Naiki H., et al. Curcumin has potent anti-amyloidogenic effects for Alzheimer's beta-amyloid fibrils in vitro. J. Neurosci. Res. 75, 742-750 (2004)
- Yang F., Lim G.P, Begum AN., et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. The Journal of biological chemistry, 280, 5892-5901 (2005)
- Ma QL., Zuo X., Yang F., et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. The Journal of biological chemistry, 288, 4056-4065 (2013)
- Garcia-Alloza M., Borrelli LA., Rozkalne A., et al. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. Journal of Neurochemistry, 102, 1095-1104 (2007)
- Lim GP., Chu T., Yang F., et al. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience 21, 8370-8377 (2001)
- Begum AN., Jones MR., Lim GP., et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J Pharmacol Exp Ther 326, 196-208 (2008)
- Mutsuga M., Chambers JK., Uchida K., et al. Binding of curcumin to senile plaques and cerebral amyloid angiopathy in the aged brain of various animals and to neurofibrillary tangles in Alzheimer's brain. The Journal of veterinary medical science / the Japanese Society of Veterinary Science, 74, 51-57 (2012)
- Anand P., Thomas SG., Kunnumakkara AB., et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol, 76, 1590-1611 (2008).
- Maiti P., Manna J., Veleri S. et al. Molecular chaperone dysfunction in neurodegenerative diseases and effects of curcumin. BioMed Research International 2014, 495091 (2014)
- Frautschy SA., and Cole GM., Why Pleiotropic Interventions are needed for Alzheimer's DiseaseMol Neurobiol, 41(2-3): 392–409 (2010)
- Lin R., Chen X., Li W., Han Y., Liu P., Pi R., Exposure to metal ions regulates mRNA levels of APP and BACE1 in PC12 cells: blockage by curcumin. Neurosci Lett. 440(3), 344-7 (2008)
- Zhang C., Browne A., Child D., Tanzi RE.. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J. Biol Chem. 285(37):28472-80 (2010).
- Xiong Z., Hongmei Z., Lu S., Yu L., Curcumin mediates presenilin-1 activity to reduce β- amyloid production in a model of Alzheimer's disease. Pharmacol. Rep. 63(5), 1101-8. (2011)
- Frautschy SA., Hu W., Kim P., Miller SA., Chu T., Harris-White ME., Cole GM., Phenolic anti- inflammatory antioxidant reversal of Aβ-induced cognitive deficits and neuropathology. Neurobiology of aging,. 22(6), 993-1005 (2001)
- Kuner P., Schubenel R., Hertel C., Beta-amyloid binds to p57NTR and activates NFkappaB in human neuroblastoma cells. J Neurosci Res. 54, 798–804 (1998)
- Gamblin TC., King ME., Kuret J., Berry RW., Binder LI., Oxidative regulation of fatty acid- induced tau polymerization. Biochemistry, 39(14), 203–210 (2000)
- Zhu X., Lee HG., Raina AK., Perry G., Smith MA., The role of mitogen-activated protein kinase pathways in Alzheimer’s disease. Neurosignals, 11, 270–281 (2002)
- Mohorko N., Repovs G., Popović M., Kovacs GG., Bresjanac M., Curcumin labeling of neuronal fibrillar tau inclusions in human brain samples. J. Neuropathol. Exp. Neurol. 69(4), 405-14 (2010).
- Mutsuga M., Chambers JK., Uchida K., Tei M., Makibuchi T., Mizorogi T., Takashima A., Nakayama H., Binding of curcumin to senile plaques and cerebral amyloid angiopathy in the aged brain of various animals and to neurofibrillary tangles in Alzheimer's brain. J. Vet. Med. Sci., 74(1):51-70 (2012).
- Ma Q., et al., β-Amyloid Oligomers Induce Phosphorylation of Tau and Inactivation of Insulin Receptor Substrate via c-Jun N-Terminal Kinase Signaling: Suppression by Omega-3 Fatty Acids and Curcumin. J. Neurosci., 29(28), 9078–9089 (2009)
- Garcia-Alloz MS., Dodwell L., Borelli A., Raju S., Backskai BJ., In vivo reduction of plaque size in APPswe/PS1D9 mice treated with curcumin (P4-342) Alzheimer’s and Dementia, 2 Suppl:S617 (2006)
- Behl C., Davis J., Cole GM., Schubert D., Vitamin E protects nerve cells from amyloid β-protein toxicity. Biochem. Biophys. Res. Commun., 186, 944–950 (1992)
- Mrakand RE., Griffin WS., Interleukin-1, neuroinflammation, and Alzheimer’s disease.Neurobiol. Aging, 22, 903–908 (2001)
- Mishra S., and Palanivelu K., The effect of curcumin (turmeric) on Alzheimer's disease: An overview. Ann. Indian. Acad. Neurol., 11(1), 13–19 (2008)
- Maiti P., and Manna J., Dietary Curcumin: A Potent Natural polyphenol for Neurodegenerative Diseases Therapy. MOJ of Anatomy & Physiology, 1(5): 00026 (2015)
- Koronyo-Hamaoui M., et al., Identification of Amyloid Plaques in Retinas from Alzheimer’s Patients and Noninvasive In Vivo Optical Imaging of Retinal Plaques in a Mouse Model Neuroimage, 54 Suppl 1:S204-17 (2011)
- Kelley BJ., and Petersen RC., Alzheimer’s Disease and Mild Cognitive Impairment. Neurol.Clin. 25(3), 577–v (2007)
- Lee WH., Loo CY., Bebawy M., Luk F., Mason RS., and Rohanizadeh R., Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century. Current Neuropharmacol, 11(4), 338-378 (2013).