Information
Journal Policies
Expression Profile of Human PARK2 Splicing Isoforms in Peripheral Blood Cells and Isolated Lymphomonocytes
Grazia Maugeri 1, Valentina La Cognata 1,2, Soraya Scuderi 1,3, Agata Grazia D’Amico 1,4, Rita Reitano 1, Salvatore Saccone 5, Concetta Federico 5, Sebastiano Cavallaro2 , Velia D’Agata*,1
2 Institute of Neurological Sciences, National Research Council, Catania, Italy.
3 Yale Child Study Center, Yale School of Medicine, New Haven, USA.
4 San Raffaele Telematic University of Rome, Italy.
5 Section of Animal Biology, Department of Biological, Geological and Environmental Sciences, University of Catania; Italy.
Copyright : © 2016 Agata VD. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Mutations in Parkin gene are responsible of 50% of cases with autosomal recessive juvenile-onset Parkinson’s disease (ARJP). Although 21 parkin alternative splice variants have been cloned so far, most of the studies have focused their attention on the full-length protein. Despite the expression of the originally cloned protein has been observed in human blood cells, there is currently no study that has investigated the expression profile of parkin isoforms in human lymphomonocyte (LMN). In the present study, we have explored the expression pattern of parkin proteins in total LMN and in specific subpopulations like T lymphocyte (CD2+), monocyte (CD14+) and B lymphocyte (CD19+).
Peripheral blood was collected from healthy volunteers (n=5). Total LMN homogenate expresses H1, H5 and H6 parkin isoforms. These data have been confirmed by immunoprecipitation analysis, by using three antibodies recognizing different domains of the full-length. In human CD2+, CD14+ and CD19+ subpopulations, two bands of ~58 and ~52 kDa molecular weight have been revealed. These proteins are distributed both in cytoplasm and nucleus, as demonstrated by fluorescence immunolocalization. The expression of parkin isoforms in human blood cells is subpopulation-specific, and is likely altered in PD patients. A future investigation of the parkin splicing profile in human blood cells could represent a useful tool for identify early non-invasive biomarkers in patients affected by ARJP.
Keywords: Parkin Protein; Alternative Splicing; T Lymphocyte; Monocyte; B Lymphocyte.
Abbreviations: ARJP: Autosomal Recessive Juvenile-Onset Parkinson Disease; LMN: Human Lymphomonocytes; UBQ: Ubiquitin-Like Domain; IBR: In-Between Ring Fingers; BSS: Balanced Salt Solution
1.Introduction
Parkin gene (also known as PARK2) is one of the largest in the human genome. It is located on the long arm of chromosome 6 (6q25.2-q27) and its mutations cause autosomal recessive juvenile-onset Parkinson's disease (ARJP) [1]. Affected patients commonly present atypical clinical symptoms, such as the onset dystonia, hyperreflexia and diurnal fluctuations [2]. Moreover, the brain of most ARJP patients with parkin mutations lacks Lewy Bodies suggesting its the involvement in the formation of these highly ubiquitinated intracytoplasmic inclusions. Accordingly, Parkin has been previously demonstrated to act as an E3 ubiquitin ligase that regulates the degradation of misfolded proteins through proteasomal pathway [3-5], and specifically it catalyzes the transfer of ubiquitin from an E2 ubiquitin-conjugating enzyme to a protein substrate. Along with the involvement in the proteasomal pathway, many other functions of parkin are currently known. It promotes the removal of damaged mitochondria via mitophagy by interacting physically with the outer mitochondrial membranes: therefore, the loss of Parkin activity causes mitochondrial dysfunction and the consequent loss of neurons in ARJPD brain [6]. Parkin reduction causes endoplasmic reticulum degeneration, another condition involved in disease progression [7]. More recently, it has been suggested that parkin acts as a tumor suppressor gene. Although, the mechanism whereby it plays this role is still unknown, a feedback between parkin and p53 has been identified [8-10]. In particular, parkin decreases p53 expression at transcriptional level whereas p53 induces an upregulation of parkin levels [11]. This balance is abolished in ARJP as a consequence of parkin mutation and loss of its ability to suppress p53 expression by contributing to exacerbated cell death in the brains of ARJP patients [11].
The multiplicity of parkin functions could be related to the transcription of different variants and consequently isoforms. To date, GenBank reports at least 26 different human PARK2 transcripts corresponding to 21 alternative splice variants, encoding a wide spectrum of protein isoforms. These isoforms present a molecular architecture and domain compositions different from the first cloned one [12]. In fact, the first cloned PARK2 transcript (presently referred as canonical) encoded a predicted protein including an N-terminal ubiquitin-like domain (UBQ), two C-terminal domains in-between ring fingers (IBR), and a cysteine-rich RING0 domain [13-15]. The UBQ domain targets specific protein substrates for proteasomal degradation, whereas IBR domains occur between pairs of ring fingers and play a role in protein quality control. The different parkin isoforms structurally diverge from the canonic one for the presence or absence of the UBQ domain and one or both IBR domains. Moreover, when the UBQ domain is present, it often differs in length from that of the canonical sequence. Interestingly, some isoforms miss all of these domains [16].
Parkin expression profile has been showed both in physiological and pathological conditions [12, 16-21]. In the central nervous system, parkin immunoreactivity has been observed in different brain areas, including substantia nigra, locus coeruleus, putamen and frontal cortex [22-25]. It has also been observed in human blood, however, little is known about the isoforms expression profile in human LMN. Sunada et al. [26] have demonstrated that, through alternative splicing mechanism, distinct parkin transcripts are expressed in different human tissues including leukocytes. A shorter and hardly detectable isoform compared to the full length was revealed in these cells. Instead, Kasap et al. [27] found a variant of about ~52 kDa molecular weight in the serum of nine patients with Parkinson disease.
In light of these evidence, in the present study, we investigated the possibility of detection of different parkin isoforms in total LMN and isolated subpopulations of a control cohort group. Our analysis revealed that human lymphmonocytes express H20, H1/H5 and H6 parkin isoforms. However, the use of more sensitive methods for the detection of these transcripts in isolated LMN subpopulation is necessary. The characterization of parkin expression profile in ARJP patients samples might allow to identify biomarkers useful in the diagnosis of this neurodegenerative disease.
2.Materials and Methods
Peripheral blood from healthy volunteers (n=5) was collected in heparin tubes, after obtaining signed informed consent. Lymphomonocytes were isolated from blood by using Ficoll-plaque plus density gradient centrifugation medium (GE Healthcare). Briefly, each heparinized blood sample was diluted with the same quantity of balanced salt solution (BSS). Then, it was layered on 3 mL of Ficoll-Plaque medium and centrifuged at 400 x g for 40 min with no brake. The buffy layer containing LMN was collected by using a sterile glass pipetteand transferred into a clean conical tube and washed 2 times with BSS.
Isolation of T lymphocyte (CD2+), monocytes (CD14+) and B lymphocyte (CD19+), was performed by using respectively Dynabeads CD2, Dynabeads CD14 and Dynabeads CD19 pan B, as specified in the instruction manuals (Invitrogen by Life Technologies). Briefly, 1 x 107 cells/mL LMN (to isolate T cells and monocytes) and 2,5 x 107 cells/mL LMN (to isolate B cells) were incubated with 25 μl pre-washed beads for 20 min on ice. Then, each sample was washed twice with isolation buffer. Cells pellet was resuspended in culture medium and was incubated at 37 °C in a humidified atmosphere with 5% CO2.
Immunoprecipitation was performed by using Dynabeads Protein A Immunoprecipitation Kit as specified in the instruction manual to isolate purified parkin isoforms (Invitrogen by Life Technologies). Briefly, 1 μg of rabbit anti-Park2 polyclonal antibody (cat n. AB5112, Millipore) or 1 μg of rabbit anti-Parkin (cat n. PAB1105, Abnova) or 1 μg of mouse anti-Parkin (cat n. SC32282, Santa Cruz Biotechnology), was conjugated with dynabeads and stored in PBS with 0.1% tween 20. To allow antigen binding, 400 μg of total LMN protein homogenate was added to each tube containing dynabeads-conjugated antibody. Each tube containing dynabeads-Ab-Ag complex was placed on magnet and supernatant was removed. The complex was resuspended in 20 µl elution buffer with 2X Laemmli buffer and processed for western blot analysis.
To determine the expression of parkin isoforms protein, western blot analysis was performed as previously described by [28]. Briefly, proteins were extracted with buffer containing 20 mMTris (pH 7.4), 2 mM EDTA, 0.5 mM EGTA; 50 mM mercaptoethanol, 0.32 mM sucrose and a protease inhibitor cocktail (Roche Diagnostics) using a Teflon-glass homogenizer and then sonicated twice for 20 sec using an ultrasonic probe, followed by centrifugation at 10.000 g for 10 min at 4 °C. Protein concentrations were determined by the Quant-iT Protein Assay Kit (Invitrogen). About 17 μg of T lymphocyte (CD2+), monocytes (CD14+), B lymphocyte (CD19+), or 50 μg of total LMN or 400 μg of immunoprecipitated homogenate was diluted in 2X Laemmli buffer (Invitrogen, Carlsbad, CA, USA), heated at 70°C for 10 min. Proteins were separated on a Biorad Criterion XT 4-15% Bis-tris gel (Invitrogen), by electrophoresis, and then transferred to a nitrocellulose membrane (Invitrogen). Blots were blocked using the Odyssey Blocking Buffer (Li-Cor Biosciences) and hybridized to each of the following antibodies: 1:1000 rabbit anti-Park2 polyclonal antibody (cat n. AB5112, Millipore), 1:500 rabbit anti-Parkin antibody (cat n. PAB1105, Abnova), 1:200 mouse anti-Parkin (cat n. SC32282, Santa Cruz Biotechnology), 1:500 rabbit anti-β-tubulin (cat n.sc-9104, Santa Cruz Biotechnology). A goat anti-rabbit IRDye 800 CWantibody, (cat #926-32211; Li-Cor Biosciences) or goat antimouse IRDye 680CW (cat #926-68020D, Li-Cor Biosciences) were used. Blots were scanned with an Odyssey Infrared Imaging System (Odyssey).
To determine the cellular distribution of parkin isoforms, immunofluorescence analysis was performed on LMN, T cells (CD2+), monocytes (CD14+) and B cells (CD19+). Briefly, cells cultured on glass cover slip were fixed in 4% paraformaldehyde in PBS (15’ at room temperature), permeabilized with 0.2% Triton X100, blocked with 0.1% BSA in PBS, and then probed with primary antibody previously described. Signals were revealed with Alexa Fluor 488 goat anti-rabbit antibody after incubation for 1.5 h at room temperature and shielded from light. DNA was counterstained with DAPI (#940110, Vector Laboratories). After a series of PBS and double-distilled water washes, the fixed cells were cover-slipped with Vectashield mounting medium (Vector Laboratories, Inc., Burlingame, CA, USA). Parkin immunoreactivity was analyzed by using confocal laser scanning microscopy (CLSM; Zeiss LSM700). Green and blue signals were detected with laser 488nm/10mW and 405nm/5mW respectively, and using the objective "PLAN-APOCHROMAT" 63X/1,40 OIL DIC M27. Each scanning was individually digitalized by a high sensitivity PMT using the following acquisition setup: Gain master: 776; digital offset: -202; digital gain: 1.0. All acquisitions were performed with ZEN-2010 software.
3.Result
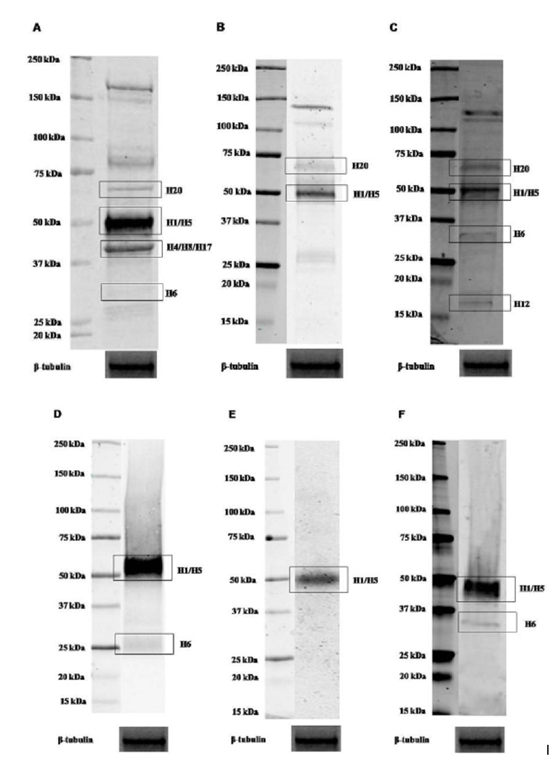
In order to identify parkin isoforms expressed in human blood cells, we used a previous published table (Table 1) that reports the predicted molecular weight of all 21 predicted parkin isoforms [16]. This table also indicates which isoform might be identified by the antibodies used in our experiments. The prediction has been performed by aligning the amino acid sequences of the isoforms with the epitope sequence recognized by each antibody. As displayed, when these sequences perfectly matched, it was indicated in the table by ‘Yes’. If the antibody recognized at least 8 consecutive amino acids, it was indicated in the table by ‘May be’. Instead, if the antibody recognized less than 8 consecutive amino acids, this was indicated in the table by ‘No’. H1 isoform is the originally cloned Parkin protein (GenBank Accession Number: BAA25751.1) and here considered as the canonical sequence.
In total LMN protein homogenate, signals corresponding to parkin proteins with a molecular weight ranging between ~58 and ~48 kDa, corresponding to H20 and H1/H5 isoforms, have been detected by AB5112, PAB1105 and SC32282 antibodies on blot (Figure. 1 A, B and C). A band of ~42 kDa molecular weight has been only observed on blot hybridized with AB5112 antibody. As predicted in Table 1, it may correspond to H4/H8/H17 isoforms (Figure. 1A). A further very faint band of ~35 kDa molecular weight, representing H6 isoform, has also been visualized by AB5112 and SC32282 antibodies (Figure. 1A and C). Moreover, a faint band of ~19 kDa, corresponding to H12 isoform, has been observed on blot by using SC32282 antibody (Figure. 1C). Unpredicted bands visualized on blots could represent aspecific signals or isoforms not identified yet. Therefore, to discriminate among specific signals, proteins have been immunoprecipitated from cell lysate by using all antibodies. As shown in Figure. 1 (D, E and F), a band of ~52 kDa has been detected. Furthermore, as previously described, a band of ~35 kDa molecular weight has also been visualized by AB5112 and SC32282 antibody (Figure. 1D and F). Instead, it is not detected the presence of the band of ~58 kDa molecular weight. As shown in Figure. 1, this might be due to low expression of this transcript in total LMN.
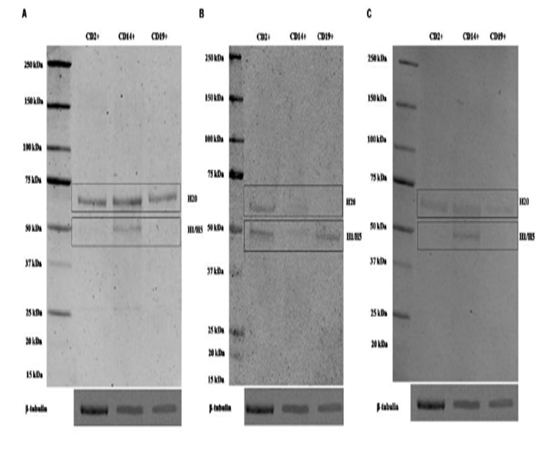
After this overall analysis, we focus our attention into the expression profile of parkin isoforms in human LMN subpopulations, including T lymphocyte (CD2+), monocyte (CD14+) and B lymphocyte (CD19+). A band of ~52 kDa molecular weight, corresponding to H1 and H5 isoforms, has been detected in all of the three subpopulation analyzed. However, the signal was more evident on the blot by using the antibody PAB1105 (Figure. 2B), whereas this band was visualized only in monocyte (CD14+) by using the other two antibodies (Figure. 2A and C). Furthermore, a band of ~58 kDa, corresponding to H20 isoform, was also revealed in all three LMN subpopulations, but the signal was most evident by using AB5112 antibody (Figure. 2A). These discordant results might depend on the different affinity of each antibody toward the various isoforms.
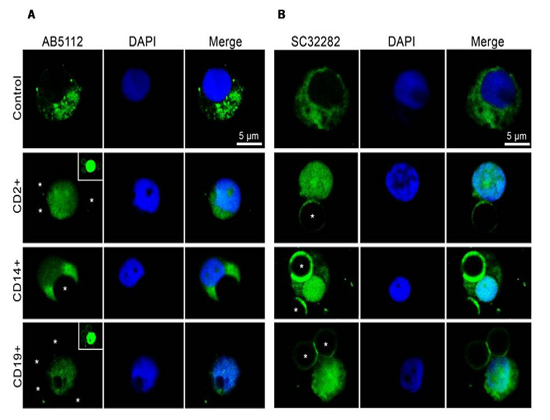
Parkin isoforms distribution in LMN subpopulations has been detected by using immunofluorescence analysis. As previously described [16], and here highlighted in Table 1, to date, there are not currently commercially available antibodies which allow to discriminate among specific isoforms. This limits the immunolocalization analysis because the detected immunofluorescence represents the combination of signals generated by the isoforms expressed in each cell. However, the data obtained have confirmed the results of western blot. In all cells, parkin is highly expressed both in cytoplasm and nucleus (Figure. 3A and B), but nuclear immunoreactivity has been predominantly revealed by SC32282 antibody (Figure. 3B). This could be explained by a higher affinity of this antibody against isoforms present in the nucleus or by its better permeation in cellular preparation.
Representative photomicrographs show parkin isoforms expression (green) in T lymphocyte CD2+, monocyte CD14+ and B lymphocyte CD19+, detected by AB5112 (A) and SC32282 (B) antibody, respectively. Nuclei were stained with DAPI (blue). Photomicrographs are representative results visualized from different fields randomly selected on slide and scanned by confocal laser scanning microscopy (CLSM; Zeiss LSM700). CD2+, CD14+, and CD19+ cells were selected using respectively Dynabeads CD2, Dynabeads CD14 and Dynabeads CD19 pan B. The dynabeads were indicated by asterisks. In the cases they were not clearly visible, a small insert at the upper right has been added (in the inserts, green signals have been brought to saturation, to show the dynabeads).
4.Discussion
In the present paper, we have described, for the first time, parkin isoforms expression both in total and LMN subpopulations such as T lymphocyte (CD2+), monocyte (CD14+) and B lymphocyte (CD19+). To date, few papers have studied parkin expression in human blood [26, 27].
Previously, Sunada et al. [26] have identified parkin splice variants in healthy human peripheral leucocytes, even if they underlined that the full-length transcript was hardly detectable in this cellular population. They observed that through alternative splicing a distinct parkin transcript is expressed in leukocytes by splicing out 3-5 exons. More recently, Kasap et al. [27] through a detailed analysis of Parkin mRNA secondary structure, found that the full-length variant contains many hairpins which might interfere with the amplification reactions. Therefore, they optimized the protocol and finally a 1.4-kb full-length park2 cDNA product was generated. They found that the protein is expressed in the peripheral leucocytes at not detectable level, while in serum, after reduction of albumin, a signal of ~52 kDa was visualized on blot. Therefore, the authors concluded that low albumin reduction fails to give a signal. This suggests that albumin hides parkin immunoreactivity. To confirm their results, they performed MALDI-TOF analysis on pieces of area cutted off from blots with a molecular weight ranging between ~50-55 kDa. This analysis revealed the presence of six different proteins with similar molecular weight.
Despite previous papers, in the present study, we have been able to detect parkin isoforms in human LMN by using consistent amount of proteins as compared to other works. To validate results, we have hybridized blot by using three antibodies that recognize different domain of the full-length protein, and their expression has been confirmed following immunoprecipitation with each antibody. Our results have shown that total LMN express high levels of H1 and H5 parkin proteins, and low levels of H6 isoform.
Parkin isoforms identified in the present study are differentially expressed in the brain of healthy or PD patients. In fact, recently, Brudek et al. [29] have demonstrated that the PARK2 TV1 variant (corresponding to H1 isoform) is significantly increased in normal substantia nigra, whereas TV3 isoform (corresponding to H6), weakly detected in control brain, is highly expressed in the cerebellar cortex of PD patients. These evidences suggest a likely correlation between parkin isoforms expression in the brain and peripheral blood, indicating this latter as an useful brain tissue surrogate [30]. Further investigations are needed to evaluate parkin isoforms expression profile in LMN of PD patients.
The H20 / H4 / H8 / H17 / H21 / H12 isoforms have been detected on blot of total LMN but not on immunoprecipitated proteins. This could be due to a low affinity of these antibodies against the various isoforms or because some proteins are weakly expressed. Furthermore, the expression of proteins with ~42 kDa molecular weight has been previously demonstrated [27].
Since total LMN include a heterogeneous cell population, in this study we have also focused our attention on some of these cells like T lymphocyte (CD2+), monocyte (CD14+) and B lymphocyte (CD19+). All of them express H20, H1 and H5 isoforms, although the presence of others isoforms at low levels, and therefore undetectable, cannot be ruled out.The presence of parkin proteins in LMN subpopulations have been confirmed by immunofluorescence analysis. Our data have suggested that they are present not only in the cytoplasm but also at the nuclear level as previously described [23, 31, 32].
The present study demonstrate that parkin isoforms are expressed in lymphomonocytes, however, more sensitive experimental protocols should be developed for their detection. It is also desirable the production of new antibodies able to selectively identify each isoform. These latter ones may allow to characterize better parkin proteins expression profile in peripheral blood cells, and may be useful as a tool for early, minimally invasive diagnoses and outcome of patients affected by ARJP.
5. Acknowledgements
This study was supported by the international Ph.D. program in Neuroscience, University of Catania, Medical School.
References
- Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N., Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998, 392 (6676), 605-8. 10.1038/33416.
- Khan, N. L.; Graham, E.; Critchley, P.; Schrag, A. E.; Wood, N. W.; Lees, A. J.; Bhatia, K. P.; Quinn, N., Parkin disease: a phenotypic study of a large case series. Brain : a journal of neurology 2003, 126 (Pt 6), 1279-92.
- Shimura, H.; Hattori, N.; Kubo, S.; Mizuno, Y.; Asakawa, S.; Minoshima, S.; Shimizu, N.; Iwai, K.; Chiba, T.; Tanaka, K.; Suzuki, T., Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nature genetics 2000, 25 (3), 302-5. 10.1038/77060.
- Imai, Y.; Soda, M.; Takahashi, R., Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. The Journal of biological chemistry 2000, 275 (46), 35661-4. 10.1074/jbc.C000447200.
- Zhang, Y.; Gao, J.; Chung, K. K.; Huang, H.; Dawson, V. L.; Dawson, T. M., Parkin functions as an E2-dependent ubiquitin- protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proceedings of the National Academy of Sciences of the United States of America 2000, 97 (24), 13354-9. 10.1073/pnas.240347797.
- Narendra, D.; Tanaka, A.; Suen, D. F.; Youle, R. J., Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. The Journal of cell biology 2008, 183 (5), 795-803. 10.1083/jcb.200809125.
- Bouman, L.; Schlierf, A.; Lutz, A. K.; Shan, J.; Deinlein, A.; Kast, J.; Galehdar, Z.; Palmisano, V.; Patenge, N.; Berg, D.; Gasser, T.; Augustin, R.; Trumbach, D.; Irrcher, I.; Park, D. S.; Wurst, W.; Kilberg, M. S.; Tatzelt, J.; Winklhofer, K. F., Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell death and differentiation 2011, 18 (5), 769-82. 10.1038/cdd.2010.142.
- Alves da Costa, C.; Checler, F., Apoptosis in Parkinson's disease: is p53 the missing link between genetic and sporadic Parkinsonism? Cellular signalling 2011, 23 (6), 963-8. 10.1016/j.cellsig.2010.10.020.
- da Costa, C. A.; Sunyach, C.; Giaime, E.; West, A.; Corti, O.; Brice, A.; Safe, S.; Abou-Sleiman, P. M.; Wood, N. W.; Takahashi, H.; Goldberg, M. S.; Shen, J.; Checler, F., Transcriptional repression of p53 by parkin and impairment by mutations associated with autosomal recessive juvenile Parkinson's disease. Nature cell biology 2009, 11 (11), 1370-5. 10.1038/ncb1981.
- Sunico, C. R.; Nakamura, T.; Rockenstein, E.; Mante, M.; Adame, A.; Chan, S. F.; Newmeyer, T. F.; Masliah, E.; Nakanishi, N.; Lipton, S. A., S-Nitrosylation of parkin as a novel regulator of p53-mediated neuronal cell death in sporadic Parkinson's disease. Molecular neurodegeneration 2013, 8, 29. 10.1186/1750-1326-8-29.
- Zhang, C.; Lin, M.; Wu, R.; Wang, X.; Yang, B.; Levine, A. J.; Hu, W.; Feng, Z., Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proceedings of the National Academy of Sciences of the United States of America 2011, 108 (39), 16259-64. 10.1073/pnas.1113884108.
- La Cognata, V.; Iemmolo, R.; D'Agata, V.; Scuderi, S.; Drago, F.; Zappia, M.; Cavallaro, S., Increasing the Coding Potential of Genomes Through Alternative Splicing: The Case of PARK2 Gene. Current genomics 2014, 15 (3), 203-16. 10.2174/1389202915666140426003342.
- Hristova, V. A.; Beasley, S. A.; Rylett, R. J.; Shaw, G. S., Identification of a novel Zn2+-binding domain in the autosomal recessive juvenile Parkinson-related E3 ligase parkin. The Journal of biological chemistry 2009, 284 (22), 14978-86. 10.1074/jbc.M808700200.
- Walden, H.; Martinez-Torres, R. J., Regulation of Parkin E3 ubiquitin ligase activity. Cellular and molecular life sciences : CMLS 2012, 69 (18), 3053-67. 10.1007/s00018-012-0978-5.
- Wauer, T.; Komander, D., Structure of the human Parkin ligase domain in an autoinhibited state. The EMBO journal 2013, 32 (15), 2099-112. 10.1038/emboj.2013.125.
- Scuderi, S.; La Cognata, V.; Drago, F.; Cavallaro, S.; D'Agata, V., Alternative splicing generates different parkin protein isoforms: evidences in human, rat, and mouse brain. BioMed research international 2014, 2014, 690796. 10.1155/2014/690796.
- La Cognata, V.; D'Agata, V.; Cavalcanti, F.; Cavallaro, S., Splicing: is there an alternative contribution to Parkinson's disease? Neurogenetics 2015, 10.1007/s10048-015-0449-x.
- La Cognata, V., D Agata V., Cavalcanti F., Cavallaro S., Parkin Alternative Splicing: Not Only Parkinsonism. ARC Journal of Neuroscience 2016, 1 (1), 9-18.
- D'Amico, A. G.; Maugeri, G.; Magro, G.; Salvatorelli, L.; Drago, F.; D'Agata, V., Expression pattern of parkin isoforms in lung adenocarcinomas. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 2015, 36 (7), 5133-41. 10.1007/s13277-015-3166-z.
- Maugeri, G.; D'Amico, A. G.; Magro, G.; Salvatorelli, L.; Barbagallo, G. M.; Saccone, S.; Drago, F.; Cavallaro, S.; D'Agata, V., Expression profile of parkin isoforms in human gliomas. International journal of oncology 2015, 47 (4), 1282-92. 10.3892/ijo.2015.3105.
- Maugeri, G.; D'Amico, A. G.; Reitano, R.; Saccone, S.; Federico, C.; Cavallaro, S.; D'Agata, V., Parkin modulates expression of HIF-1alpha and HIF-3alpha during hypoxia in gliobastoma-derived cell lines in vitro. Cell and tissue research 2016, 364 (3), 465-74. 10.1007/s00441-015-2340-3.
- Shimura, H.; Hattori, N.; Kubo, S.; Yoshikawa, M.; Kitada, T.; Matsumine, H.; Asakawa, S.; Minoshima, S.; Yamamura, Y.; Shimizu, N.; Mizuno, Y., Immunohistochemical and subcellular localization of Parkin protein: absence of protein in autosomal recessive juvenile parkinsonism patients. Annals of neurology 1999, 45 (5), 668-72.
- Huynh, D. P.; Scoles, D. R.; Ho, T. H.; Del Bigio, M. R.; Pulst, S. M., Parkin is associated with actin filaments in neuronal and nonneural cells. Annals of neurology 2000, 48 (5), 737-44.
- Huynh, D. P.; Scoles, D. R.; Nguyen, D.; Pulst, S. M., The autosomal recessive juvenile Parkinson disease gene product, parkin, interacts with and ubiquitinates synaptotagmin XI. Human molecular genetics 2003, 12 (20), 2587-97. 10.1093/hmg/ddg269.
- Dagata, V.; Cavallaro, S., Parkin transcript variants in rat and human brain. Neurochemical research 2004, 29 (9), 1715-24.
- Sunada, Y.; Saito, F.; Matsumura, K.; Shimizu, T., Differential expression of the parkin gene in the human brain and peripheral leukocytes. Neuroscience letters 1998, 254 (3), 180-2.
- Kasap, M.; Akpinar, G.; Sazci, A.; Idrisoglu, H. A.; Vahaboglu, H., Evidence for the presence of full-length PARK2 mRNA and Parkin protein in human blood. Neuroscience letters 2009, 460 (3), 196-200. 10.1016/j.neulet.2009.05.079.
- D'Amico, A. G.; Scuderi, S.; Maugeri, G.; Cavallaro, S.; Drago, F.; D'Agata, V., NAP reduces murine microvascular endothelial cells proliferation induced by hyperglycemia. Journal of molecular neuroscience : MN 2014, 54 (3), 405-13. 10.1007/s12031-014-0335-2.
- Brudek, T.; Winge, K.; Bredo Rasmussen, N.; Bahl Czarna, J. M.; Tanassi, J.; Klitmoller Agander, T.; Hyde, T. M.; Pakkenberg, B., Altered Alpha-Synuclein, Parkin, and Synphilin Isoform Levels in Multiple System Atrophy Brains. Journal of neurochemistry 2015, 10.1111/jnc.13392.
- Sullivan, P. F.; Fan, C.; Perou, C. M., Evaluating the comparability of gene expression in blood and brain. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 2006, 141B (3), 261-8. 10.1002/ajmg.b.30272.
- D'Agata, V.; Grimaldi, M.; Pascale, A.; Cavallaro, S., Regional and cellular expression of the parkin gene in the rat cerebral cortex. The European journal of neuroscience 2000, 12 (10), 3583-8.
- D'Agata, V.; Zhao, W.; Pascale, A.; Zohar, O.; Scapagnini, G.; Cavallaro, S., Distribution of parkin in the adult rat brain. Progress in neuro-psychopharmacology & biological psychiatry 2002, 26 (3), 519-27.