Information
Journal Policies
Attenuation of Drug-Induced Nephrotoxicity in Rats
Using Zingiberofficinale Roscoe
Nadia A. Mohamed1*, Jihan Hussein1, Safinaz E EL-Toukhy1, Rehab A. Mohamed1, Zakaria El-Khayat1
Copyright : © 2018 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Drug nephrotoxicity is still a problem for patients who taken drugs for elongated periods or permanently. So the purpose of this study is to ameliorate this effect by using medicinal plant (ginger) for its antioxidant effect. Wistar strain male albino rats were divided into 4 groups Group 1 treated with saline. Group 2 was administered with one dose of cisplatin (ip) (12 mg/kg) or till the level of the nephrotoxicity. Groups 3 dosed by ginger (310 mg/kg) (po) for one month. Groups 4 reached to the nephrotoxicity level then they were treated with the same dose of ginger for one month. At the end of the experiment the blood collected to evaluate serum urea and creatinine by spectrophotometer. Urinary 8- hydroxyguanosine (8-OHdG) evaluated by HPLC. Oxidative stress markers [nitric oxide (NO), Malondialdehyde (MDA), reduced glutathione (GSH) and paraoxinase -1 (PON1) evaluated in kidney tissue homogenate. Also the kidney tissue was examined histopathologically. Treatment with ginger revealed significant reduction in MDA levels, 8-OHdG and NO levels but promoted activities of GSH and PON 1 in comparison with cisplatin-treated group (p < 0.001). Also, it improves urea and creatinine levels as well as kidney histopathology. The ginger has protective effect on the kidney; we recommend for more studies with longer periods of administration or different doses to enhance amioleration of drug toxicity.
drug nephrotoxicity – ginger – oxidative stress – cisplatin,Nephrology
1. Introduction
Humans take a number of drugs for medication, it is especially guaranteed that all useful drugs will produce undesirable effects, but some can produce positively dangerous effects [1]. It is renowned that many drugs can be transformed in the body to multiple metabolites which stimulate therapeutic as well as toxicologic responses. Chemically, these metabolites are inactive and their effects were appeared by joining reversibly with active sites in tissues. In some cases these drugs turned into chemically reactive metabolites in the body where either uncouple conjoined processes in cells or combine covalently with many tissue biopolymers, as glycogen, DNA, RNA, and protein. It has become increasingly obvious that chemically reactive metabolites cause many types of earnest toxicity, such as mutagenesis, carcinogenesis, fetotoxicity and cellular necrosis [2]. Therefore, there is a great need to ameliorate the toxic effect of drugs that the patient is forced to get them for elongated time or permanently. So, in this study we elucidate a new way to get these treatments without falling in drug toxicity. Cisplatin is a platinum-based drug, which is the most effective anti-neoplastic drug, applied as treatment for testicular, bladder, ovarian, lung, cervical, and neck cancers [3]. The cytotoxic influence of cisplatin is resulted mainly from its overlapping with DNA, via the formation of covalent adducts between definite DNA bases and platinum compound [4], despite its medical damage, the treatment with cisplatin has been coordinated with many toxic side effects as nephrotoxicity, hepatotoxicity and cardiotoxicity. It has been documented that oxidative stress via the formation of reactive oxygen species, reduces antioxidant defense system as antioxidant enzymes and non-enzymatic molecules, reduced glutathione are the main disorders of toxicity of cisplatin [5]. Ginger belongs to family-Zingiberaceae, it has been cultivated for many years as a spice and for medicinal uses [6]. It has been as an important ingredient in Chinese herbal medicines for the remediation of rheumatism, diabetes, nausea, stroke, asthma, toothache, gingivitis and vomiting [7]. The extracts of ginger are rich in gingerols and shagaols which display anti- inflammatory, anti-oxidant and anti-carcinogenic properties by ‘‘in vivo’’ and ‘‘in vitro’’ systems [8]. This work was designed to assess the pre-emptive effect of orally administered ginger extract versus cisplatin-induced nephrotoxicity in rats.
2. Material And Methods
Cisplatin [cis-diamminedichloroplatinum(II)] was supplied from Sigma-Aldrich Chemical Company. The dose of cisplatin was calculated according to body surface area. In the following study, we used a dose of 12 mg/kg, by converting an equivalent dose of 75 mg/m2 (for humans) to a rat equivalent dose in mg/kg [9]. By using the following formula: Human equivalent dose (mg/kg) / human km = animal equivalent dose (mg/kg) / animal km, km constant (Coefficient factor for species); km (coefficient factor for humans) =37 and km (Coefficient factor for rats) =6.
The ginger was supplied from Arab Company for Pharmaceuticals and Medicinal Plants (MEPACO, Cairo, Egypt) (tablet form, 400mg, human dose is 1:3 g/day) [10]. In our study, we utilized a dose equivalent to 3 g/day equivalent for an average human body weight of 60 kg and converted it to a rat equivalent dose in mg/kg, which was 310 mg/kg/day [9].
Wistar strain male albino rats (180 ± 20 g), was obtained from the Animal House, National Research Centre (NRC). They were housed in clean polypropylene cages and kept in a controlled temperature room and given a standard diet and water ad libitum through the experimental period. The experiments were carried out according to guidelines and protocol approved by the Institutional Animal Ethics Committee.
The rats were divided into groups of 10 animals each. Rats were given saline (po) (group 1) for 30 successive days and were injected (ip) with one ml saline on day 20 of the experiment. The group [2] was the cisplatin group. The rats were injected (ip) with a single dose of 12 mg/kg cisplatin. The group [3] was ginger group (310 mg/kg) daily for 30 successive days (po). The group [4] was the cisplatin and ginger group. The rats in this group received ginger extract (310 mg/kg) daily for 20 successive days (po), and then on day 20 of ginger extract administration each rat was injected intraperitoneally with a single dose of 12 mg/kg cisplatin. At the end of the experiment the animals were euthanized by decapitation. Blood, urine and kidney tissues were collected. Tissues were fixed in 10% neutral buffered formalin for histopathological examination or homogenized for the estimation of kidney parameters [11].
Kidneys were removed quickly and washed with iced normal saline to remove blood cells, plotted on filter paper and frozen at -80 °C until used for estimation of other biochemical parameters. The frozen tissues were cut into pieces and homogenized in phosphate buffer (pH 7.4), then centrifuged at G-value (3200g) for 15 minutes at 4 °C and the supernatant was removed for chemical parameters estimation [12].
Serum urea was performed according to the method of [13] and serum ceatinine was performed according to the kinetic method of Young [14]. The kits were supplied from Spectrum Company. Lipid peroxidation was assayed by measuring the level of malondialdehyde (MDA) in serum using the method of Ruiz- Larrea et al., [15]. Nitric oxide (NO) was determined using Griess reagent, according to the method reported by Moshage et al., [16] and GSH by Ellman’s method [17]. The arylesterase activity of paraoxonase was measured spectrophotometrically in supernatants using phenylacetate as a substrate. In this assay, arylesterase/paraoxonase catalyzes the cleavage of phenyl acetate, resulting in phenol formation. The rate of phenol formation is measured by monitoring the rise in absorbance at 270 nm at 25 ºC. The working reagent consisted of 20 mM Tris/HCl buffer, pH 8.0, containing 1 mM CaCl2 and 4 mM phenyl acetate, as the substrate. Samples diluted 1:3 in buffer were added and the change in absorbance was recorded following a 20-s lag time. Absorbance at 270 nm was taken every 15 s for 120 s using UV Spectrophotometer [18,19].
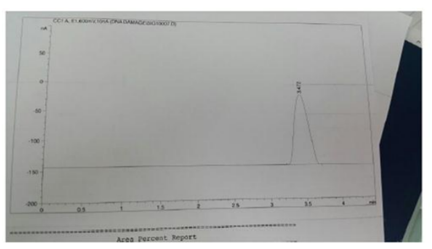
The urinary 8-hydroxyguanosine (8-OHdG) analysis was performed in accordance to the method of Kim et al.[20] by extracting 8-OHdG from 1 ml urine then the eluents were dried under ultrapure N2 stream and reconstituted in 5 ml deionized water. Sample and the different concentrations of the standard were injected in HPLC. The concentration of urinary 8-OHdG was calculated from the standard curve and divided on the urinary creatinine. Urinary creatinine was performed by kinetic method of Larsen [21].
The column of HPLC was C18 (260 × 4.6, particle size 5 μl) and the mobile phase was acetonitrile/methanol/phosphate buffer (25/10/965) v/v. Phosphate buffer was made by dissolving 8.8 g of potassium dihydrogen phosphate in one liter deionized water and pH was adjusted at (3.5). Then the buffer was filtered 2 times before using at a flow rate of 1 ml/min by using electrochemical detector with cell potential of 600 mV [22].
For microscopic evaluation kidneys were fixed in 10% neutral buffered formalin then they were dehydrated in ascending series of ethanol, cleared in zylene and embedded in paraffin wax. Sections 5 μm thickness was prepared using a microtome stained with hematoxylin and eosin (H&E) and examination under a light microscope [23].
The data was expressed as mean ± SE. The distribution of the data was verified to be normal using tests of normality (SPSS version 6). Statistical significance was tested by one way analysis of variance (ANOVA) followed by Bonferroni post hoc analysis.
3. Results
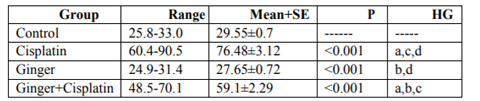
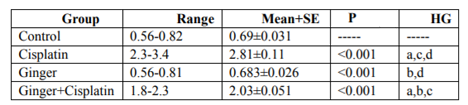
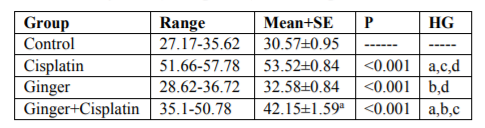
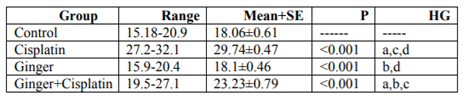
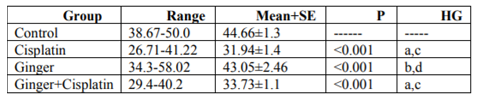
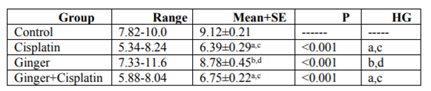
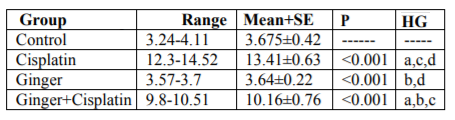
In the study, the mean values of serum urea also creatinine levels have not changed in ginger group in comparison with the control group elucidate its safety, where in cisplatin group, the mean value of serum urea and creatinine significantly increased in comparison with the control group. While, ginger in the nephrotoxicity group significantly reduced in both urea also creatinine (Table1, 2).
NO, MDA and 8-hydroxyguanosine were significantly elevated in cisplatin group, but GSH and PON1 were decreased significantly in comparison to control group elucidate the enhancement of oxidative stress, however, these levels were improved after treatment with ginger (Tables 3-7) Figure (1-3).
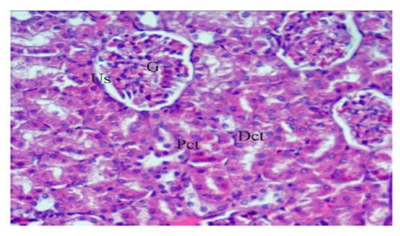
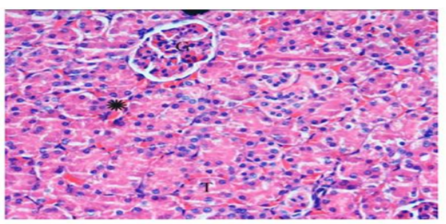
The sections of control group displayed normal renal glomeruli enclosed by urinary space and normal distal, proximal and convoluted tubules (Figure.4).
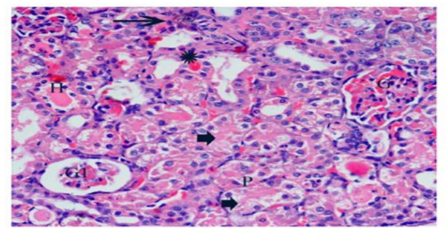
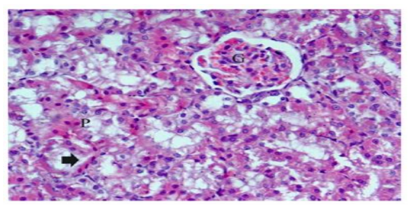
Histological examinations of cisplatin group exhibited many changes as degenerative affecting glomeruli, epithelial cells of tubules, and in the interstitium. Marked atrophy of some glomerular tufts and large Bowman’s space were also observed. The renal sections showed marked tubular degeneration, necrosis, dilatation, hyaline cast, with pyknoticneucli, apoptotic bodies were apparently. Loss of inner brush border lining proximal convoluted tubules was observed. In the interstitium of the renal cortical regions, it shows focal infiltration of mononuclear inflammatory cells which were accompanied with hemorrhage (Figure.5). In the group received ginger only displayed renal tissues appeared nearly normal structure (Figure.6). In the group treated with cisplatin and ginger revealed moderate improvement, however histology of kidney tissue showed atrophy in some glomeruli escorted with mild necrosis and congestion. Also, the tubular cells still have degeneration changes (Figure.7).
4. Discussion
The following work was performed to elucidate the nephro-protective potentials of ginger after induction of toxicity by cisplatin. The renal damage caused from cisplatin counts on the dose or treatment duration. The study was issued with high level dose of cisplatin (12 mg/kg/ body weight, i.p) for induction of nephrotoxicity which related to the dosage of cisplatin which used in clinical study. As the experimental model is important due to cisplatin could be toxic in humans and resulted in renal failure [24]. In the current study, cisplatin elevated serum creatinine and urea, this in accord with Abeer et al.,[25] who mentioned that chronic renal injuries by cisplatin intoxication were related with urea and creatinine increases which are indicators of kidney injury. This value was reduced significantly in ginger treated group in comparing with control and cisplatin groups. Acute kidney injury stimulated by induction of cisplatin in experimental rats causes a significant decrease in the glomerular filtration rate and an elevation in serum urea as well as serum creatinine [26]. These results elucidate the amelioration of kidney function markers, such as serum urea and serum creatinine levels, after treatment with ginger in cisplatin-treated rats [27]. Oxidative damage is commonly linked with the formation of highly reactive OH which resulted in severe oxidative damage of the cell's components like proteins, lipids as well as DNA [28]. In current study, the lipid peroxidation markers; NO and MDA were enhanced in cisplatin group as reported earlier [29]. The enhancement in lipid peroxidation could be attributed to enzymatic and non-enzymatic antioxidants of defense systems. With regard to the enzymatic or non-enzymatic antioxidant levels, the present study revealed significant reduction in glutathione and PON1 in cisplatin-treated rats. These findings were in agreement with Sarawoot et al., [3] who reported the same disturbance in the antioxidant levels after cisplatin induction of damage. The reduction of glutathione level concerning its role as antioxidant gives an additional support of the elevation of free radicals involved in renal toxicity by cisplatin. In addition, reductive dehalogenation of cisplatin with P450 enzyme system towards to the highly reactive trichloromethyl radical inhances the lipid peroxidation process, as it is supposed as the most important mechanism in the pathogenesis of renal damage [30]. Moreover, these metabolites can interact with sulfhydryl groups of glutathione and protein thiols which alter the redox status of cells [29]. Therefore, GSH is considered as an important defense against lipid oxidative damage in the kidneys to eliminate the hydrogen peroxide, hydroxyl radicals and peroxyl formed. Treatment with ginger recorded improvement of the GSH, PON1, NO and MDA. Protective effects of ginger against cisplatin which induced oxidative stress could be refered to the high level of polyphenol compounds (6-gingerol and its derivatives), that have a high antioxidant activity [31]. These compounds could scavenge the free radicals of cisplatin formed through P450 enzyme system and diminish the oxidative damages. Ginger may also impair cisplatin-mediated lipid peroxidation by decreasing the production of free radical derivatives. Administration of ginger alone to the animals showed insignificant changes in all the antioxidant parameters confirming the potency of the ginger extracts as anti-free radicals-producer. Also there was an increase in 8-OHdG in urine. These findings were in conformity with the results of preceding studies as the increase in urinary 8-OHdG excretion was linked with a decrease in 8-OhdG-positive cells in kidney, proposing that urinary 8-OHdG is not derived from tubular cells. Since Cisplatin is responsible for a slow cellular damage in neurons [32], increasing urinary 8-OHdG could lead to DNA damage in non-renal tissues. The increase in urinary 8-OHdG and the dissociation from the number of 8-OHdG-positive tubular cells propose that urinary 8-OHdG is not a good marker of oxidative DNA damage in renal tubules in CDDP-induced acute renal failure [33]. The histopathological observations of Cisplatin-induced nephrotoxicity displayed degenerative affecting glomeruli, epithelial cells of tubules, and in the interstitium. Marked atrophy of the glomerular tufts was observed and showing large Bowman’s space as an evident. The renal sections showed marked tubular degeneration, necrosis, dilatation, hyaline cast, with pyknoticneucli, apoptotic bodies were apparently. Loss of inner brush border lining proximal convoluted tubules was observed. In the interstitium of the renal cortical regions, there was focal infiltration of mononuclear inflammatory cells which was associated with hemorrhage. These histopathological changes were parallel to previous reports [34].
5. Conclusions
The administration of ginger extract markedly protected against the cisplatin- nephrotoxicity in which was indicated by the histological improvements in the kidney architecture. These results are a good indicator on the chemoprotective and antioxidative activities of ginger. This direct radical scavenging activity could effectively deplete the reactive electrophilic metabolites resulted from the metabolic activation of cisplatin in the proximal tubules of the kidneys. In present study administration of ginger alone did not show any side effects, which indicates its non-toxic nature.
References
- Liebler DC and Guengerich FP. Elucidating mechanisms of drug-induced toxicity. Nat Rev Drug Discov 2005; 4:410–420.
- David E G, Armen H T and Ehrin J A. Principle of pharmacology. The pathophysiologic basis of drug therapy.4 thed; 2016. ISNB 978-1-60831-270-2.
- Sarawoot P, Chuchard P, Dtsadee C, and Prasit S. Amelioration of Cisplatin-Induced Nephrotoxicity in RatsbyCurcumin and α-Tocopherol. Tropical Journal of Pharmaceutical Research 2013; 12 (6): 973-979.
- Mohamed A, Al-Kahtani AM, Abdel-Moneim OM, and Mohamed AE. Hemin Attenuates Cisplatin-Induced Acute Renal Injury inMale Rats. Oxidative Medicine and Cellular Longevity2014; Article ID 476430, 9 pages.
- Domitrovi c R, Cvijanovi c O, Pernjak-Pugel E M, Skoda L, Mikeli c, and ˇZ. Crncevi’c- Orli’c. Berberine exerts nephron protective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis,” Food and Chemical Toxicology2013; 62: 397–406
- Park EJ, and Pezzuto JM. Botanicals in cancer chemoprevention, Cancer Metastasis Rev2002; 21: 231–255.
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, Pharmac- ological and toxicological properties of ginger (Zingiber officinale roscoe). Food Chem. Toxicol 2008;46: 409–420.
- Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food and Chemical Toxicology2002;40: 1091–1097
- Reagan-Shaw S, Nihal M and Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008; 22:659–661
- Ozgoli G, Goli M and Simbar M. Effects of ginger capsules on pregnancy, nausea and vomiting. J Altern Complement Med 2009; 15:243–246.
- Madway W, Prier LE and Wilkinson JS. (1969): A textbook of veterinary clinical pathology; The Willims and wilkingco. Battimore 1969 page 201
- Manna F, Ahmed HH, Estefan SF, Sharaf HA, and Eskander EF. Saccharomyces cerevisiaeintervension for relieving flutamide-induced hepatotoxicity in male rats. Pharmazie 2005; 60: 689-695.
- Young D., S. (2005): Effect of drugs on clinical laboratory tests.5th Ed; AACCpress, Washington D.C. 2005. Page 300
- Young D., S. (2007): Effect of preanalytical variables on Clinical Laboratory Tests, 3rd; Washington D.C. 2007. Page 707
- Ruiz-Larrea MB, Leal AM and Liza M. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids1994; 59:383–388.
- Moshage H., Kok B. and Huizenga J.R Nitrite and nitrate determination in plasma: a critical evaluation. Clin Chem1995; 41:892– 896.
- Ellman GL: Tissue sulfhydryl groups. Arch Biochem 1959; 82:70–77.
- Higashino K, Takahashi Y and Yamamura Y. Release of phenylacetate esterase from liver microsomes by carbon tetrachloride. Clin Chim Acta 1972; 41:313–320.
- Watson AD, Berliner JA and Hama SY. Protective effect ofhigh density lipoprote in associated paraoxonase. Inhibition of thebiological activity of minimally oxidized low density lipoprotein.J Clin Invest 1995; 96:2882–2891
- Kim M, Moon H, Hong S. Determination of urinary 8-hydroxy-2-deoxy guanosine as a DNA damage marker. Am. Clin. Lab 2001; 42-45.
- Larsen K Creatinine assay by a reaction-kinetic principle. Clin.Chim. Acta1972; 41:209-217.
- El-Khayat Z, Rasheed W, Ramzy T, Hussein J, Agaiby M, Morsy S,Morsy F, Shaffie N. Protective effect of garlic oil against liverinjury in experimental animals, J. Med. Plants Res 2010; 4(22):2359-2369.
- Xiupu S, Sha L, Huijuan M, Changrun G, Dongli Q, Fei L Chunfeng Z and Zhonglin Y. Hesperidin Prevents Retinal and Plasma Abnormalities in Streptozotocin-Induced Diabetic Rats. Molecules 2012; 17: 12868-12881.
- Marcio J D, Gisele G, Renata G, Expedito L S, Janaina S D, Rui MB, Alciléia N Y and Ciomar AB. Ginger Essential Oil Ameliorates Cisplatin-Induced Nephrotoxicity in Mice. Tropical Journal of Pharmaceutical Research 2013; 12 (6): 959-965
- Abeer F A, Mohamed SE, Dalia M E and Doaa IE. Histological study on the role of ginger against cisplatininduced testicular toxicity in albino rats The Egyptian Journal of Histology2013; 36:312-320
- Rjiba-Touati K, Boussema IA., Belarbia A, Achour A and Bacha H. Protective effect of recombinant humanerythropoietin against cisplatin-induced oxidativestress and nephrotoxicity in rat kidney. IntJToxicol 2011; 30: 510-517.
- Mazaheri S, Nematbakhsh M, Bahadorani M, Pezeshki Z,Talebi A, Ghannadi AR and Ashrafi F. Effects offennel essential oil on cisplatin-induced nephrotoxicity in ovariectomized rats. Toxicol int 2013; 20: 138-145
- Mckillop IH and Schrum LW. Alcohol and liver cancer. Alcohol 2005; 35(3): 195-203.
- Manal AH, Sanaa A A and Nagy SE. (2012): Therapeutic Potential of Ginger against Renal Injury Induced by Carbon Tetrachloride in Rats. The Scientific World Journal 2012; Article ID 840421, 12 pages.
- Khan MR, Rizvi W, Khan GN, Khan RA and Shaheen S. Carbon tetrachloride-induced nephrotoxicity in rats: protective role of Digeramuricata,” Journal of Ethnopharmacology 2009; 122 (1): 91–99.
- Stoilova I, Krastanov A, Stoyanova A, Denev P and Gargova S.0Antioxidant activity of a ginger extract (Zingiberofficinale). Food Chemistry 2007; 102 (3) 764–770.
- McKeage MJ, Hsu T, Screnci D, Haddad G and Baguley BC. Nucleolar damage correlates with neurotoxicity induced by different platinum drugs. Br J Cancer 2001; 85: 1219– 1225
- Hua Z, Akihiko K, Takehiko M, Hideo Y, Yoshihide F, Tatsuo Y, Katsuhiko Y, Satoru T, Hiroyuki M and Akira H. Urinary marker for oxidative stress in kidneys in cisplatin-induced acute renal failure in rats. Nephrol Dial Transplant 2006; 21: 616–623.
- Gonzalez R, Borrego A, Zamora Z, RomayC, Hernández F, Menendez S, Montero T and Rojas R. Reversion by ozone treatment of acute nephrotoxicity induced by cisplatin in rats. Mediat. Inflamm 2004; 13(5-6): 307–312.