Information
Journal Policies
Mammalian Target of Rapamycin Inhibitors Monotherapy: Efficacy in Renal Transplant. Three Years Later
Antonio Franco1*,Patricio Mas-Serrano2,Noelia Balibrea1,David Rodriguez1,Iris Garcia1,Javier Perez Contreras1
2.Departments of Pharmacy Hospital General Universitario de Alicante, Spain.
Copyright : © 2018 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
We developed a protocol on m-Tor monotherapy three years ago that avoids high pill burden and could improve adherence and outcome. In a three years extension study we examined the evolution of 83 of 98 recipients who was on m-TOR monotherapy at the end of the previous study.
At the final of the follow up time, 74 months, 11 recipient more have dropped out .Finally 72 of 98 recipients (73,4%) remained on the protocol.
Renal function slightly decreased at the end of the study, but it was still better than the one registered before starting the protocol. On the other hand, proteinuria was higher. There was no change in levels of sirolimus, but everolimus levels fell a significant degree during the extended period.
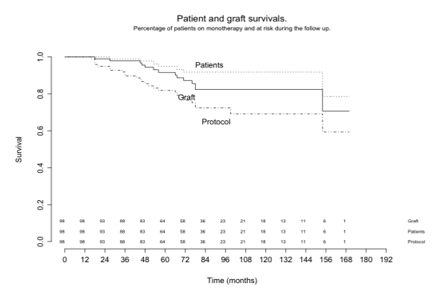
The use of erythropoietin decreased while there was no change in body weight, angiotensin-converting enzyme inhibitors, hypotensive drugs or lipid-lowering drugs use and patients with diabetes. No case of non adherence was detected. The rates of recipient and graft survivals were 97, 7% and 94.4% at 4 years and, 91.7% and 87, 2% at 6 years, while the percentages of patients on monotherapy were 85.5% and 76.6 % respectively.
This extended study supports the previous reported data of safety and efficacy on m-Tor monotherapy as immunosuppressive agent in selected renal transplant recipients.
renal transplant, m-TOR inhibitors, monotherapy, protocol,Nephrology
1. Introduction
Mammalian target of rapamycin inhibitor (m-Tor) have no nephrotoxic effects and can avoid cell proliferation and migration by blocking the intracellular signaling pathway that regulates the proliferation of activated Tcells [1]. These cells have a potential protective role against renal graft dysfunction, improve patient cardiovascular profiles and reduce the rate of cancer by also reducing rates of angiogenesis [1,2]
Besides, non adherence is a major risk factor for graft failure [3]. Multiple daily dosing can contribute to lack adherence [4]. We then developed a protocol on m-Tor monotherapy that avoids high pill burden and could improve adherence and outcome. Evidences on the topic could justify the conversion to this protocol on m-Tor monotherapy of all patients who have no contraindication for it.
We reported a serie of 98 low immunological risk renal transplant recipient on m-Tor monotherapy and concluded the safety and efficacy of monotherapy with m-Tor as immunosuppressive agent in selected renal transplant recipients [5].
We decided to extent this study three years more to confirm our previous experience.
2. Material And Methods
This is a three years extension study of a previous prospective study of renal transplant recipient on m-Tor monotherapy. Briefly, the previous study was a prospective, simple arm, observational one in which 98 patients were followed up for 46 months on m-Tor monotherapy. Fifteen recipient dropped out of the study (15.3%): 8 patients (8.2%) had to change their immunosuppression regime due to complications and 7 (7.1%) lost their grafts as a result of chronic rejection (4 cases) or death (3 cases). At the end of follow up, 83 of 94 recipients (84.6%) remained on monotherapy. Renal function improved in a significant degree while proteinuria decrease was not significative. After starting the protocol, the use of eritropoyetin, angiotensin-converting enzyme inhibitors (ACE)/angiotensin receptor blockers (ARB), and other hypotensive agents increased [5].
After the completion of the study, follow up was continued in periodic visits. The data reported in the current article correspond to the data obtained at the third year after the publication of the serie. The parameters monitored were: trough m-Tor levels, renal function measured by serum creatinine and the Glomerular rate filtration by MDRD-4 ( modification on diet in renal disease study equation), biopsy proven acute rejection episodes, proteinuria, use of eritropoyetin, lipid-lowering drugs , ACE/ ARB, hypotensive agents, body weight, incidence of diabetes, recipients on protocol and patient and graft survivals.
The plasma concentrations of sirolimus and everolimus were determined using immunoassay techniques (ACMIA Siemens(R) on auto-analyser Dimension XPand® platform and QMS Thermo Fisher (R) on Indiko(R) platform, respectively). The sensitivity limit of these techniques was 2ng/dl and 1, 5ng/dl, respectively. Compliance was evaluated through a personal interview and by measuring drug levels.
3. Statistical Analysis
Continuous variables are expressed as mean and 95% CI, or as median and interquartile range, depending on the distribution of the data. For the description of categorical variables, the number and percentage of patients per response category have been used.
We compared variables between groups using ANOVA tests in continuous variables or the equivalent for non-parametric variables, depending on the inherent characteristics for each study variable, and McNemar tests for analysing categorical variables from related samples.
Recipient, graft survival, and patients on m-TOR monotherapy were studied using Kaplan-Meier survival curves. All statistical analyses were carried out using SPSS statistical software, version 19.0. In all statistical tests applied to the study variables, a statistical significance (α) of 0.05 was considered.
4. Results
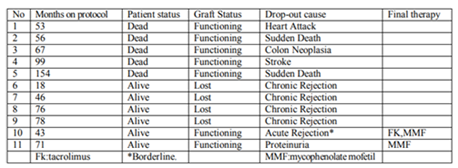
A total of 83 of 98 initial recipients were enrolled in the extension study. Demographic data of our cases, causes of the previous drop out and the percentage of patients on sirolimus or everolimus has been previously reported [5]. The medium follow up was 74(p25-p75: 49-91,) months,(range 18-153) . At the end of the follow up time 11 recipients more have dropped out of the study due to different causes, as showed in table 1, rising the total drop-out number to 26 ,26.5% of the initial population. Finally 72 out of 98 (73, 4%), recipients remain on the protocol.
Renal function slightly decreased during the extended period in the patients who were on the protocol at the end of the study, serum creatinine increased from 1.5 (95%CI: 1.1-1.8) mg/dl to 1.6 (95%IC: 1.1-2.0) mg/dl (p= 0.005) and GRF by MDRD decreased from 45.1(95%IC: 36.6-64.6) to 40.4 (95%IC: 31.2- 61.5) ml/min (p=0.003), but it was better than before starting the protocol,1.8 (95%CI: 1.2-2.6) mg/dl (p=0.0012) and 38.2 (95%IC: 25.2-55.4) ml/min (p=0.027). Proteinuria was higher, increasing from 132 (p25-p75: 46-356) to 213 (p25-p75: 90-652) mg/24h (p= 0.005) and was the main cause to drop out from the study for of one patient (case 11).
There was no change in the trough levels of sirolimus, from 10.4ng/ml (95% CI: 9.7-11.1) to 9.7ng/ml (95% CI: 9.0-10.5) p=0.17. On the other hand, the levels of everolimus, significantly decreased from 7,8ng/ml (95% CI: 6.6 – 9.1ng/ml) to 6, 0 ng/ml (95% CI: 5.0 – decreased but there was no change in body weight (72 (95%IC: 65-81) to 74(95%IC: 64- 7.0ng/ml), p= 0.035. 80) kg), p=0.069, percentage of recipients with ACE)/ ARB (50.0% to 41.7 %; p=0.180), hypotensive drugs (from 76.1% to 69.7%; p=0.172) use of lipid-lowering drugs (77.3% to 73.6%; p=0.238) and patients with DM (31.7% a 31.9; p: 0.203). No case of nonadherence was recorded during the follow up.
The rates of patient and graft survivals were 97,7% and 94.4% at 4 years and , 91.7% and 87,2% at 6 years, while the percentages of patients on monotherapy after 4 and 6 years were 85.5% and 76.6 % respectively (figure 1).
5. Discussion
It is well known that renal function declines gradually after transplantation. In a large cohort of stable renal transplants, Gill reported that GFR decreased an average of 1, 66 ml/mn per year over a follow up, similar to ours, of 5.7 years [6]. Thus, our recipients maintained a stable renal function and, better than expected, higher than before starting the protocol, perhaps due to the lack of nephrotoxicity[1].
Proteinuria was the side effect responsible for the removal of one patient from the protocol (case11 Table 1) and increased a significant degree during the extended period. Although it was believed that proteinuria was the consequence of Calcineurin inhibitors removal, it seems to be an independent effect of m-TOR, due to glomerular hyper-filtration [7]. ACE/ARB can reduce proteinuria in renal transplant recipients[8], but only partially. In spite of the fact that a high percentage of our patients (41, 7%) receive ACE/ARB, we reported a significant increase of proteinuria.
The use of concomitant medication did not change and we reduced the percentage of recipients with erythropoietin in a significant degree. These data point out a stabilization or even an improvement of the clinical condition of our patients on protocol. Sellares [3] has recently reported non adherence as a major risk factor for graft failure. Multiple daily dosing can contribute to lack adherence [4]. Our protocol on m-Tor monotherapy avoids high pill burden and could improve adherence and outcome, As a matter of fact we have recorded no cases of non adherence during the follow up (medium 74 months).
One patient in our extension study developed a mild episode of acute rejection, borderline (case 10 Table 1), who responded to a change in immunosuppression. We consider that our rate of acute rejection is low, since our patients were on monotherapy, and Guirado has reported incidences of 0.6, 1, 1 and 0, 4% during the first, second and third year in a large cohort of stable renal recipients converted from twice-daily to once-daily tacrolimus[9] reflecting that increased experience with m-TOR has provided important guidance for clinical use [10].
There are very few data about the trough levels on m-Tor monotherapy. Pinto reported a level of 9, 6 ± 3,3 ng/ml of sirolimus at the end of his study[11], similar to ours, and has no experience in everolimus. We found a reduction of the trough levels of everolimus in the extended period, based on clinical experience, without adverse effects which could be applied to sirolimus too.
Most of our recipients who dropped out from the extended study (cases 1-5 Table 1) lost their grafts due to death with a functioning kidney. At the present time, this is the most frequent cause of transplant failure reported in the literature [12]. These data seems to recommend a possible reduction in immunosuppression dose since our protocol is potent enough to avoid rejection.
Three-quarters of our patents, 72 out of 98 (73, 4%), remained on the protocol at the end of the extended follow-up time (Figure 1), which was
quite long. Besides, their renal function decreased less than the usual degree seen in other renal transplant patients [6] and was better than before enrolling on the protocol. There were only 26 drop outs of the total population and all of them were late. These results confirm the absence of nephrotoxic effects and the adequate and well tolerated immune suppression achieved with mTOR [13].
6. Conclusion
Our study is observational, for which conclusions are limited due to the absence of a control group, but the experience is useful for daily clinical practice. This extended study supports the previous reported data of safety and efficacy on m-Tor monotherapy as immune suppression in selected renal transplant recipients.
Acknowledgments
This project received financial support from Pfizer Spain in connection with the development of this manuscript.
References
- Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, Barr ML. Immunosuppression: evolucion in practice and trends, 1994-2004. Am J Transplant; 6:1111-31. (2006)[Pubmed]
- Meier-Kriesche HU, Schold JD, Srinivas TR. Kapplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant ;4:378-383(2004).[Pubmed]
- Sellares J, de freitas DG, Mengel M et al .Understanding the causes of kidney transplant failure .the dominant role od antibody mediated rejection and non adherence. Am J Transplant ;12:388-399. (2012).[Pubmed]
- Saini SD, SchoenfeldP,Kaulback K, Dubinsky MC .Effect of medication dosing frecuency on adherence in chronic diseases.Am J Mahag Care;15:e22-e23. (2009).[Pubmed]
- Franco A, Más-Serrano P,Perez Contreras J ,Jimenez L., RodriguezD,Olivares JMammalian target of rapamycin inhibitors monotherapy: efficacy in renal transplant .Trans proc ; 47,2364_2367 (2015).[Pubmed]
- Gill JS,Tonelli M, Mix CH et al The change in allograft function among long-term kidney transplant recipients.j Am SocNephrol ;14:1636-1642. (2003).[Pubmed]
- Jaurina A, Campistol JM, Piera C, Diekmann F, Campos B, Campos N, et al. Conversion from calcineurin inhibitors to sirolimus in chronic allograft disfunction. Changes in glomerular haemodinamics and proteinuria. Nephrol Dial Transplant; 2:488-493. (2006).[Pubmed]
- Mandelbrot D, Alberú J, Barama A, MarderB, Tedesco-Silva H, Flynn A,Healy C et al. Effect of Ramipril on Urinary Protein Excretion in Maintenance Renal Transplant Patients Converted to Sirolimus.Am J Transplant;14(3S) Abstract 2213.( 2014)
- Guirado L, Burgos D,Cantarell C, Fernandez A, Franco A et al Medium-Term renal function in a large cohort of stable kidney transplant renal patients converted from twice-daily to once-daily tacrolimus. Transplantation direct ;1.e24;doi:10.1087( 2015)
- Sánchez-Fructuoso AI1, Ruiz JC, Pérez-Flores I, Gómez Alamillo C, Calvo Romero N, Arias M.Comparative analysis of adverse events requiring suspension of mTOR inhibitors: everolimus versus sirolimus.Transplant Proc. Oct;42(8):3050-3052. ( 2010) [Pubmed]
- Pinto JR, Arellano Torres EM, Franco A, Morales JM, Ruiz JC, Diekmann F, et al. Sirolimus monotherapy as maintenance immunosuppression: a multicentre experience. Transpl Int;23:307-312( 2010).[Pubmed]
- Yu, M., Kim, Y.C., Lee, J.P. et al. Death with graft function after kidney transplantation: a single-center experience Clin Exp Nephrol https://doi.org/10.1007/s10157-017-1503-9. (2017).
- Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (m-Tor) in renal disease. J Am SocNephrol;20:2493-2502. (2009).[Pubmed]