Information
Journal Policies
Obesity-Related Pathways to Chronic Kidney Disease
Luís Belo1*, Sandra Ribeiro1, Maria do Sameiro-Faria2,3, Vasco Miranda3, Alice Santos-Silva1
2 Pediatric Nephrology Department of CMIN (Centro Materno-Infantil do Norte),Porto Hospital Centre, Porto, Portugal
3 Frenesius Medical Care, Nephrocare Maia, SA, Portugal
Copyright : © 2016 Luís B. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Obesity is a major public health problem and an important risk factor for the development of chronic kidney disease (CKD). Several factors have been pointed to explain this association, but the exact mechanisms are not fully understood. In this work we focused on possible direct and indirect mechanisms underlying CKD development in obese patients. The need of potential interventions to lower CKD development in these patients is also highlighted.
Keywords: Obesity, chronic kidney disease, risk factors, prevention, weight loss.
Obesity and chronic kidney disease (CKD) are well recognized public health problems increasing worldwide. Cohort studies demonstrated that obesity is associated with higher risk of CKD [1, 2], and its progression may lead to end-stage renal disease (ESRD). This association appears to be particularly enhanced in case of clustering of risk factors (e.g. metabolic syndrome) [3, 4]. The pathogenesis of CKD in obesity is not fully understood, but excessive body fat can lead to kidney disease via several (direct or indirect) mechanisms.
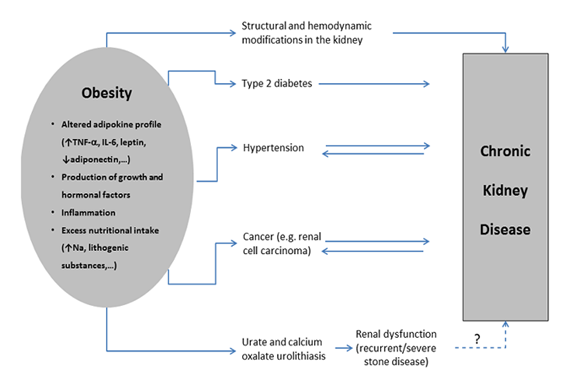
In Portugal, as in many other countries, the main causes of CKD include diabetes, arterial hypertension, chronic glomerulonephritis and polycystic kidney disease [5]. Other causes include: reflux nephropathy, obstructive uropathy, hereditary nephropathy, nephrolithiasis, chronic interstitial nephritis, autoimmune diseases (e.g. systemic lupus erythematosus), repeated urinary infections and cancer. Interestingly, some of the etiologies leading to CKD are also considered obesity-related comorbidities (Figure 1), in particular diabetes and hypertension. Actually, it is well recognized that obesity is a major risk factor for diabetes and hypertension, the two most common etiologies for CKD [5]. Obesity is also associated with focal and segmental glomerulosclerosis, urolithiasis and cancer.
An altered adipokine profile is observed in obesity, mainly increased pro-inflammatory substances (including tumor necrosis factor-alpha and interleukin (IL)-6) and decreased adiponectin (an adipokine with important protective properties, namely anti-inflammatory and antidiabetic) that may underlie many of the associating comorbidities, including diabetes, hypertension and CKD itself. Furthermore, excess of body fat may trigger the production of growth factors, such as transforming growth factor (TGF)-, and hormones, such as aldosterone, that induce kidney damage [6, 7]. Indeed, TGF- is a known pro-fibrotic factor that may contribute to renal fibrosis and to increase the renal expression of inflammatory cytokines, such as IL-6, further aggravating renal damage [8]. Obesity associates with structural and hemodynamic modifications in the kidney (e.g. mesangial expansion of the glomerulus, glomerular hyperfiltration and hypertension), that may ultimately lead to glomerulosclerosis and CKD (Figure.1). Actually, obesity is a recognized cause of focal segmental glomerulosclerosis [9].
Obesity increases the risk of urate and calcium oxalate urolithiasis [10, 11], mainly due to increased nutritional intake that raises lithogenic substances, such as uric acid and oxalate. Although this type of urolithiasis represents a benign condition, it can (rarely) lead to CKD, particularly in severe or recurrent cases. In addition, it seems that subjects with a history of nephrolithiasis present a decrease in renal function compared to those without [12].
Obesity also increases the risk of some types of cancer, namely renal cell carcinoma (RCC) [13]. It is unknown how obesity increases RCC incidence, but it may involve an abnormal adipokine secretion [14]. It is important to emphasize that cancer and CKD are tightly interconnected, as cancer can cause CKD (via direct or indirect ways, namely through the adverse effects of therapeutic interventions), but CKD may work as a risk factor for cancer [15]. Furthermore, both cancer and CKD share common risk factors, namely obesity (as previously referred) and toxins.
The association of obesity with development of CKD and the increasing prevalence of both diseases all over the world [16] raises the importance of intervention studies on weight reduction and, consequently, on obese-related comorbidities, including CKD and later ESRD. Previous work from our group showed that ESRD patients present several modifications in classical and non-classical cardiovascular risk factors – including inflammatory markers (e.g. increase in IL-6 and C-reactive protein levels) and lipid profile (e.g. increased proportion of oxidized low-density lipoprotein particles) [17] – and that weight reduction can improve insulin sensitivity, lipid profile and blood pressure in obese patients [18, 19]. Recent data also suggest that weight-loss may retard or even reverse the progression of CKD, mainly at early stages of the disease [20, 21].
It is particularly important to highlight the progressively increasing prevalence of obesity at pediatric ages, when healthy habits should be stablished. Actually, a high birth weight increases the risk of both obesity and type 2diabetes later in life [22], and an increment in diabetes prevalence has been observed in the past decades all over the world [23]. Thus, to combat this public health problem, preventive measures should be drawn and put into practice as early in life as possible. This is even more relevant if we consider that in the context of obesity, loss of kidney function seems to be exacerbated with aging [24].
In synthesis, obesity is an epidemic disease that has potential to evoke CKD (another emerging epidemic) through a variety of mechanisms, either direct or indirect. Increased physician awareness of obesity-related comorbidities is needed to screen, early diagnose and prevent the progression of kidney disease in obese patients.
References
- Wang Y., Chen X., Song Y., Caballero B. and Cheskin L.J., Association between obesity and kidney disease: a systematic review and meta-analysis, Kidney Int. 73(1), 19 (2008).
- Tsujimoto T., Sairenchi T., Iso H., Irie F., Yamagishi K., Watanabe H., Tanaka K., Muto T. and Ota H., The dose-response relationship between body mass index and the risk of incident stage ≥3 chronic kidney disease in a general japanese population: the Ibaraki prefectural health study (IPHS), J Epidemiol.24(6), 444 (2014).
- Panwar B., Hanks L.J., Tanner R.M., Muntner P., Kramer H., McClellan W.M., Warnock D.G., Judd S.E., Gutiérrez O.M., Obesity, metabolic health, and the risk of end-stage renal disease, Kidney Int. 87(6), 1216 (2015).
- Singh A.K., Kari J.A., Metabolic syndrome and chronic kidney disease. Curr Opin Nephrol Hypertens. 22(2), 198 (2013).
- Sociedade Portuguesa de Nefrologia, Gabinete de Registo 2015,
www.spnefro.pt/noticias/2016/129_gabinete_registo_2015 [accessed on 18/05/2016]. - Kawarazaki W., Fujita T., The role of aldosterone in obesity-related hypertension, Am J Hypertens. 29(4), 415 (2016).
- Stenvinkel P., Zoccali C., Ikizler T.A., Obesity in CKD - what should nephrologists know?, J Am Soc Nephrol. 24(11), 1727 (2013).
- Ribeiro S., Garrido P., Fernandes J., Vala H., Rocha-Pereira P., Costa E., Belo L., Reis F., Santos-Silva A., Renal risk-benefit determinants of recombinant human erythropoietin therapy in the remnant kidney rat model - hypertension, anaemia, inflammation and drug dose, Clin Exp Pharmacol Physiol. 43(3), 343 (2016).
- Praga M., Hernández E., Morales E., Campos A.P., Valero M.A., Martínez M.A., León M., Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis, Nephrol Dial Transplant. 16(9), 1790 (2001).
- Siener R., Glatz S., Nicolay C., Hesse A., The role of overweight and obesity in calcium oxalate stone formation, Obes Res. 12(1), 106 (2004).
- Tarplin S., Ganesan V., Monga M., Stone formation and management after bariatric surgery, Nat Rev Urol. 12(5), 263 (2015).
- Gillen D.L., Worcester E.M., Coe F.L., Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III, Kidney Int. 67(2), 685 (2005).
- Hakimi A.A., Furberg H., Zabor E.C., Jacobsen A., Schultz N., Ciriello G., Mikklineni N. et al., An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma, J Natl Cancer Inst. 105(24), 1862 (2013).
- Choi S.H., Chun S.Y., Kim T.H., Kwon G., Identifying the emerging role of adipokine as a diagnostic and prognostic biomarker of renal cell carcinoma. Urol Oncol. 1078 (2016).
- Stengel B., Chronic kidney disease and cancer: a troubling connection, J Nephrol. 23(3), 253 (2010).
- Pippias M., Stel V.S., Abad Diez J.M., Afentakis N., Herrero-Calvo J.A., Arias M., Tomilina N., et al., Renal replacement therapy in Europe: a summary of the 2012 ERA-EDTA Registry Annual Report., Clin Kidney J. 8(3), 248 (2015).
- do Sameiro-Faria M., Ribeiro S., Costa E., Mendonça D., Teixeira L., Rocha-Pereira P., Fernandes J., Nascimento H., Kohlova M., Reis F., Amado L., Bronze-da-Rocha E., Miranda V., Quintanilha A., Belo L., Santos-Silva A., Risk factors for mortality in hemodialysis patients: two-year follow-up study, Dis Markers. 35(6), 791 (2013).
- Aires L., Silva G., Martins C., Marques E., Lagoa M.J., Ribeiro J.C., Rêgo C., Nascimento H., Pereira P.R., Santos-Silva A., Belo L., Mota J., Exercise intervention and cardiovascular risk factors in obese children. Comparison between obese youngsters taking part in a physical activity school-based programme with and without individualised diet counselling: the ACORDA project, Ann Hum Biol. 43(3), 183 (2016).
- Nascimento H., Costa E., Rocha S., Lucena C., Rocha-Pereira P., Rêgo C., Mansilha H.F., Quintanilha A., Aires L., Mota J.., Santos-Silva A, Belo L., Adiponectin and markers of metabolic syndrome in obese children and adolescents: impact of 8-mo regular physical exercise program, Pediatr Res. 76(2):159 (2014).
- Hou C.C., Shyu R.S., Lee W.J., Ser K.H., Lee Y.C., Chen S.C., Improved renal function 12 months after bariatric surgery. Surg Obes Relat Dis. 9(2), 202 (2013).
- Chang A., Greene T.H., Wang X., Kendrick C., Kramer H., Wright J., Astor B., Shafi T., Toto R., Lewis J., Appel L.J., Grams M., The effects of weight change on glomerular filtration rate. Nephrol Dial Transplant. 30(11), 1870 (2005).
- Johnsson I.W., Haglund B., Ahlsson F., Gustafsson J., A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes. 10(2), 77 (2015).
- Global report on diabetes. World Health Organization, Geneva, 2016.
- Lu J.L., Molnar M.Z., Naseer A., Mikkelsen M.K., Kalantar-Zadeh K., Kovesdy C.P., Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol. 3(9), 704 (2015).