Information
Journal Policies
On the Combined Effect of Aspirin and Statins on the Mononuclear Cells' Immune Function: Are Two Better than One?
Hanna Bessler*, Ph.D, Meir Djaldetti, M.D
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Purpose: The idea behind the present investigation was to evaluate the effect of aspirin, and two statins - pravastatin (hydrophilic) and atorvastatin (hydrophobic) either one alone or in combination, on the capacity of human PBMC for cytokine production.
Methods: PBMC were incubated without or with LPS in the absence (0) or presence of each one of the followings: pravastatin, atorvastatin (50 µM) or aspirin 10 µg/ml and jointly with aspirin and one of the statins. The supernatants were tested for cytokines using ELISA kits.
Results: Atorvastatin caused inhibition of TNFα production by PBMC and enhanced the secretion of IL -1β and IL-6.The secretion of TNFα, IL -1β or IL-6 was not affected by pravastatin or aspirin. IL-2, IFN γ and IL-10 secretion by PBMC was inhibited by atorvastatin and was further reduced when aspirin was added. IL -1ra secretion was slightly enhanced by pravastatin or aspirin and by the combination of both.
Conclusion: Although all three drugs may affect cytokine production by human PBMC, albeit to different degrees, aspirin applied together with statins and particularly with atorvastatin may alter the effect of either drug alone in controlling inflammatory processes. This observation favor the jointly use of both drugs for obtaining better clinical outcome.
Abbreviations: PBMC -peripheral blood mononuclear cells, PBS -phosphate buffered saline, FBS-fetal bovine serum, LPS-lipopolysaccharide, PMA-phorbolmyristate acetate, IL-interleukin, TNF-tumor necrosis factor, IFN- interferon, DMSO-dimethyl sulphoxide, CM-complete medium
Aspirin, atorvastatin, pravastatin, cytokines, peripheral blood mononuclear cells.
1. Introduction
The history of certain drugs advances parallel to human development - acetylsalicylic acid being an appropriate example. More often known as aspirin, it served as an efficient analgesic and antipyretic for centuries. The milestones starting from use of willow leaves containing salicin against fever and swelling, till the massive introduction of aspirin into the pharmacological armamentarium have been vividly described by Wick [1]. With increasing experience it became obvious that aspirin exerts pleiotropic properties including anti-platelet, anti-thrombotic, anti-inflammatory and even anti-cancer activities [2-6]. It is well accepted that the anti-inflammatory properties of aspirin are due to its capacity to inhibit cyclooxygenase (COX) functions [7]. Therefore it is widely implemented in cardiology, as chronic inflammation of the vascular endothelium concurrently with risk factors develops to atherosclerosis with serious complications such as coronary involvement and even death [8]. In addition to its numerous pharmacological activities research has shown that aspirin is an active immunomodulating agent [7, 9]. It has been reported that aspirin affects the phagocytic capacity of the macrophages, induces apoptosis and modulates cytokine production. Peripheral blood mononuclear cells (PBMC) incubated with aspirin produce IFNγ more than threefold compared to controls [10].Considering the close relation between chronic inflammation, immune responses and development of atherosclerosis, the use of agents that may affect the chain of events linked with those processes might be of great pharmacological and clinical value. In this sense aspirin with its great variety of activities is a valuable pharmacological asset. However, development of atherosclerosis depends on a number of risk factors the highly important one being hypercholesterolemia. Therefore, efforts have been made to develop cholesterol lowering drugs resulting in synthesis of statins known as 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors [11]. Research has shown that in addition to their lowering cholesterol capacity and prevention of cardiovascular diseases, statins exert immunomodulatory properties such as anti-inflammatory activities [12]. Modulation of inflammatory cytokine production and inhibition of malignant cell proliferation [13, 14]. Enhanced phagocytosis and decreased apoptosis of human peripheral blood cells [15], as well as antioxidant and antithrombotic effects [16]. In a previous study it has been shown that there is a difference between the activity of hydrophobic and hydrophilic statins at least when some functions of PBMC, such as phagocytosis and apoptosis have been evaluated [15]. Klener et al. [17] have reported that human monocytes treated with hydrophobic statins promoted secretion of a number of inflammatory agents, among them TNFα, IL-1β and IL-8, while the hydrophobic pravastatin lacked the capacity to induce inflammatory responses. Considering the similar immune properties of aspirin and statins and their common administration in the clinic the aim of the study was to examine their joint effect on cellular proliferation and the capacity of PBMC for cytokine production applying aspirin with the hydrophilic statin-pravastatin and the hydrophobic one –atorvastatin.
2. Materials and Methods
Atorvastatin and pravastatin were kindly provided by Teva, Pharmaceutical Industries Ltd., API Division, Petah-Tiqva, Israel. Atorvastatin calcium was dissolved in dimethyl sulphoxide (DMSO, Sigma, Israel) and pravastatin in phosphate buffered saline (PBS). A stock solution of 5 mM of each statin was prepared in the appropriate diluent, and further dilutions were made in medium. The statins were added to the cell cultures at a final concentration of 50 µM. Aspirin (Acetylsalicilic acid Sigma, Israel) was dissolved in absolute ethanol at 100 mg/ml. Dilutions were prepared in complete medium, and the pH was adjusted to 7.0 using 1N NaOH. Further dilutions were carried out in complete medium. Aspirin was added to the cultures at a final concentration of 10μg/ml. Control cultures were incubated either with medium or with ethanol or DMSO at fina l concentrations of 0.2% and 1% corresponding to the concentrations added with the statins.
Buffy coats obtained from donors’ blood were purchased from Magen David Adom, Blood Services Center in Israel after signing an informed consent containing a written agreement that blood components not suitable for therapeutic needs may be used for medical research. PBMC were separated by Lymphoprep-1077 (Axis-Shield PoC AS, Oslo, Norway) gradient centrifugation. The cells were washed twice in phosphate buffered saline (PBS) and suspended in RPMI-1640 medium containing 1% penicillin, streptomycin and nystatin, 10% fetal bovine serum (FBS, Biological Industries, BeithHaemek, Israel) and was designated as complete medium (CM).
The effect of statins and aspirin on PBMC proliferation was determined using XTT proliferation assay kit (Biological Industries, Beith Haemek, Israel). Briefly, 0.1 ml aliquots of PBMC (105/ml of CM) were added to each one of 96 well plates and incubated for 24 hrs in the absence or presence of pravastatin or atorvastatin (50µM), with aspirin (10µg/ml) or with a combination of one of the statins and aspirin at concentrations as indicated. At the end of the incubation period the cells were stained according to the manufacturer’s instructions. The plates were incubated for 2-4 hrs.at 37oC in a humidified incubator containing 5% CO2 and the absorbance was measured at 450 nm using ELISA reader.
2x106 MC suspended in 1 ml of CM were incubated in 24 well plates (Nunc, Thermo Fisher Scientific, Paisley PA4 9RF, UK) without or with pravastatin, atorvastatin or aspirin, or with a combination of one of the statins and aspirin added at the onset of the cultures at final concentrations as indicated. Cultures were incubated without or with 50 ng/ml of lipopolysaccharide (LPS, E. coli, Sigma) to determine the effect of all substances on TNFα, IL-1β, IL-6, IL-1ra and IL-10 and with PMA 1µg/ml and ionomycin 0.5µg/ml (Sigma, Israel) for IL-2 and IFNγ secretion. Cultures were incubated for 24 hrs at 37oC in a humidified atmosphere containing 5% CO2. At the end of the incubation period, the culture media were collected, the cells were removed by centrifugation at 200g for 10 min and the supernatants were kept at -70oC until assayed for cytokine content.
The concentration of cytokines in the supernatants was tested using ELISA kits specific for human cytokines (Biosource International, Camarillo, CA) as detailed in the guide-line provided by the manufacturer. The detection level of all cytokines was 30 pg/ml.
Paired t-test was used to compare between the level of cytokines produced following incubation with the various drugs and that found in control cultures. In addition, it was applied to compare between cells incubated with one of the drugs with those incubated with one of the statins and aspirin jointly. Probability values of p < 0.05 were considered as significant. The results are expressed as mean ± SEM of six experiments using cells from different blood donors.
3. Results
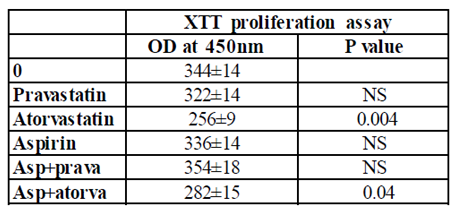
PBMC incubated for 24 hrs with either aspirin or pravastatin, or a combination of both drugs had no effect on cell proliferation. On the other hand, incubation of PBMC with atorvastatin caused 25% inhibition of cell proliferation (p=0.004), whereas incubation with both aspirin and atorvastatin caused 18% inhibition (p=0.04) that did not differ significantly from the reduction induced by atorvastatin alone.
0.1 ml aliquots of PBMC (105/ml of CM) were added to each one of 96 well plates and incubated for 24 hrs in the absence (0) or presence of pravastatin, atorvastatin (50µM), or aspirin (10µg/ml) and with a combination of one of the statins and aspirin at concentrations as indicated. At the end of the incubation period the cells were stained according to the manufacturer’s instructions. The plates were incubated for 2-4 hrs at 37oC in a humidified incubator containing 5% CO2 and the absorbance was measured at 450 nm using ELISA reader. The results are expressed as the Mean±SEM of 6 experiments. P value represents statistically significant difference from cell incubated without addition of either statins or aspirin (0).
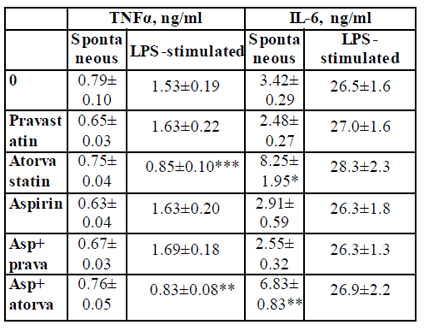
TNFα: The secretion of TNFα by non-stimulated PBMC was not affected by 24 hrs. of incubation with either pravastatin, atorvastatin or aspirin added separately or in a combination of aspirin and one of the statins. In addition, TNFα production by LPS-stimulated PBMC was not affected when the cells were incubated with aspirin. However, LPS-induced secretion of TNFα was inhibited by 45% upon incubation with atorvastatin (p < 0.003) or with a combination of atorvastatin and aspirin p < 0.01, Table 2).
2x106 PBMC suspended in 1 ml of CM were incubated for 24 hrs without (spontaneous) or with LPS 100ng/ml in the absence (0) or presence of each one of the followings: pravastatin, atorvastatin (50 µM) or aspirin 10 µg/ml and with a combination of aspirin and one of the statins. At the end of the incubation period supernatants were collected and tested for cytokines using ELISA kits. The results are expressed as Mean±SEM of 6 experiments. Asterisks represent statistically significant difference from cells incubated without drugs (0) (*p<0.05; **p<0.01; ***p<0.001).
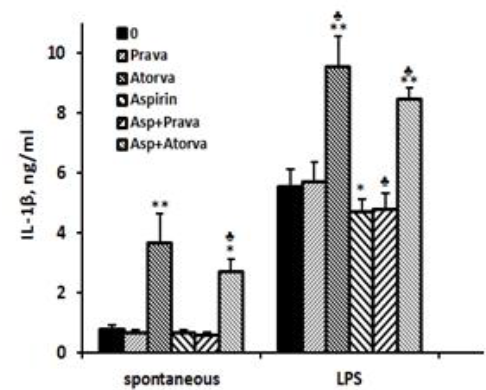
IL-1β: The spontaneous secretion of IL-1β by PBMC was 4.7 times higher (p < 0.01) when the cells were incubated with atorvastatin alone, and 3.5 times higher (p < 0.01) upon incubation with atorvastatin and aspirin compared with cells incubated without drugs, the difference between the two combinations being statistically significant (p=0.007). The secretion of IL-1β by non-stimulated PBMC was not affected by incubation with pravastatin or aspirin alone or added together. IL-1β production by LPS-stimulated PBMC was reduced by 16% upon incubation with aspirin and by aspirin plus pravastatin (p < 0.05) and was enhanced by 72% following incubation with atorvastatin (p < 0.001). LPS-stimulated PBMC incubated with aspirin and atorvastatin together caused 52% increase in IL-1β production (p < 0.005), a value significantly lower from that caused by atorvastatin alone (p < 0.01, Figure1).
2x106 PBMC suspended in 1 ml of CM were incubated for 24 hrs without (spontaneous) or with LPS 100ng/ml in the absence (0) or presence of each one of the followings: pravastatin, atorvastatin (50 µM) or aspirin 10 µg/ml, and with a combination of aspirin and one of the statins. At the end of the incubation period supernatants were collected and tested for cytokines using ELISA kits. The results are expressed as Mean±SEM of 6 experiments. Asterisks represent statistically significant difference from cells incubated without the drugs (0) (*p< 0.05; **p< 0.001). represents statistically significant difference from cells incubated with statin or aspirin alone (p< 0.05).
IL-6: IL-6 production by non-stimulated PBMC was 2.4 and 2.0 times higher following incubation with atorvastatin alone or combined with aspirin, respectively (p < 0.05). No significant difference was found between the two experimental settings. The spontaneous secretion of IL-6 was not affected by aspirin or pravastatin alone or added together. There was no effect of either of the drugs added separately of jointly on the production of IL-6 by LPS-stimulated PBMC (Table2).
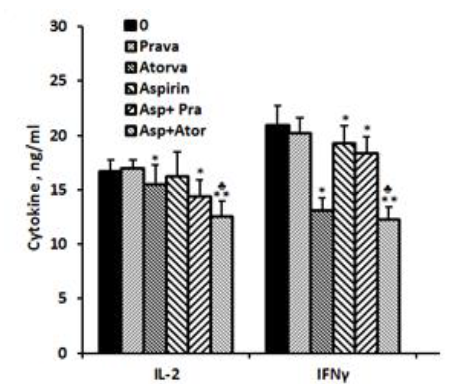
IL-2.: No detectable amounts of IL-2 could be found in supernatants obtained from non-stimulated PBMC incubated for 24 hrs without or with aspirin, statins or both. The production of IL-2 by PMA/ionomycin prompted PBMC was not affected when cells were incubated with aspirin or pravastatin alone. However when added together, the production of IL-2 was reduced by 14% (p< 0.005). In addition, atorvastatin caused 7% reduced secretion of IL-2 when added alone to PMA/ionomycin stimulated PBMC (p < 0.05) and 25% inhibition when added together with aspirin (p< 0.001). The difference between the two combinations was statistically significant (p< 0.005, Figure 2).
IFNγ: The spontaneous secretion of IFNγ by PBMC was not affected by incubation with aspirin or with one of the statins separately or jointly with aspirin. However, IFNγ production by PMA/ionomycin stimulated cells was inhibited by 37% (p=0.002) and 8% (p=0.027) following incubation with atorvastatin or aspirin alone, respectively, and by 41% (p=0.001) when added together. The inhibition in IFNγ secretion caused by incubation with both aspirin and atorvastatin was significantly more manifested as compared to each one added separately (p< 0.002). Incubation of PMA/ionomycin stimulated PBMC with pravastatin had no effect on IFNγ production. However added together with aspirin it caused 12.5 inhibition (p< 0.05, Figure 2).
2x106 PBMC suspended in 1 ml of CM were incubated for 24 hrs with PMA/ionomycin (1 µg/ml and 0.5µg/ml respectively) in the absence (0) or presence of each one of the followings: pravastatin or atorvastatin (50 µM), aspirin 10 µg/ml and with a combination of aspirin and one of the statins. At the end of the incubation period supernatants were collected and tested for cytokines using ELISA kits. The results are expressed as Mean±SEM of 6 experiments. Asterisks represent statistically significant difference from cells incubated without drugs (0) (*p< 0.05; **p < 0.001). represents statistically significant difference from cells incubated with statin or aspirin.
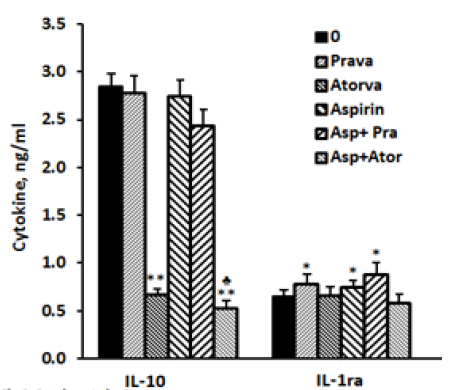
IL-10: The secretion of IL-10 by non-stimulated PBMC was 25% higher when they were incubated for 24 hrs with atorvastatin (p< 0.05).Addition of aspirin did not change significantly the production of IL-10 compared to control cells. Incubation with pravastatin or aspirin separately or jointly had no effect on IL-10 production. The secretion of IL-10 by LPS-stimulated PBMC was inhibited by 74% when atorvastatin was added alone (p< 0.001) and was further reduced by 81.5% (p< 0.001) when it was added with aspirin (p< 0.05) as compared with cells incubated with atorvastatin alone (Figure 3).
IL-1ra: The spontaneous production of IL-1ra was not affected by incubation with pravastatin, atorvastatin or aspirin added separately or combined. Its secretion by LPS induced cells was not affected by atorvastatin alone or together with aspirin. However, addition of pravastatin or aspirin to LPS stimulated PBMC caused 20% and 15% enhancement in IL-1ra secretion (p< 0.05) and was further increased to 35% (p< 0.03) when added together, but did not differ significantly from that caused by pravastatin alone (Figure3).
2x106 PBMC suspended in 1 ml of CM were incubated for 24 hrs with LPS 100 ng/ml in the absence (0) or presence of each one of the followings: pravastatin or atorvastatin (50 µM) or aspirin 10 µg/ml, and with a combination of aspirin and one of the statins. At the end of the incubation period supernatants were collected and tested for cytokines using ELISA kits. The results are expressed as the Mean±SEM of 6 experiments. Asterisks represent statistically significant difference from cells incubated without drugs (0) (*p< 0.05; **p< 0.001). represents statistically significant difference from cells incubated with statin only (p< 0.05).
4.Discussion
The presented results indicate that atorvastatin exerted a marked inhibition of PBMC proliferation while neither aspirin nor pravastatin alone affected that cell activity. The combination of aspirin with atorvastatin did not alter this function, further attesting that aspirin has no effect on PBMC proliferation at least at the present laboratory setup. Incubation of human monocyte-derived dendritic cells with simvastatin (hydrophilic) or atorvastatin (hydrophobic) inhibited their capacity to induce T cell proliferation [18]. It has been firmly established that statins may control inflammatory responses via different mechanisms, one of them being activation of mononuclear cells’ immune functions. Investigations revealed that atorvastatin may promote differentiation of classically activated pro-inflammatory M1 monocytes to alternatively activated anti-inflammatory M2 monocytes [19]. The results hereby presented indicate that aspirin, atorvastatin and pravastatin applied alone influenced in a different way the capacity for cytokine production by PBMC alongside a marked dependency on the activation status of the cells. Aspirin increased the secretion of the anti-inflammatory cytokines IL-10 and IL-1ra and inhibited that of the pro-inflammatory IFNγ. Pravastatin incubated with PBMC did not affect the production of either of cytokines examined, except for an increased secretion of the anti-inflammatory cytokine IL-1ra observed with LPS induced cells only. On the other hand, atorvastatin promoted IL-1β production by both stimulated and unstimulated PBMC, whereas the production of IL-6 was increased only by unstimulated PBMC. At the same experimental conditions the production of TNFα and IFNγ was decreased only when the cells were activated with LPS or PMA respectively. Interestingly, the secretion of IL-10 was slightly increased by unstimulated cells, while those promoted by LPS showed marked inhibition of this cytokine production. It has been reported that statins may trigger immune responses. Thus, mice given atorvastatin and rosuvastatin showed a decreased production of IL-6 and TNFα [20]. A reduced expression of TNFα, IL-1β, and IL-18 has been observed following incubation of PBMC with atorvastatin [21, 22] and of TNFα, IFNγ, IL-12 after incubation with pravastatin [23, 24]. Similar activities characterized by inhibited production of pro-inflammatory cytokines have been observed with PBMC from hypertensive patients treated with atorvastatin [25]. Likewise to statins, aspirin expresses anti-inflammatory properties. Lipopolysaccharide activated human monocytes showed dose- dependent inhibition of TNFα synthesis following incubation with aspirin [26]. Aspirin administered to healthy volunteers caused increased production of both IFNγ and IL-2 [27]. Incubation of PBMC with both aspirin and atorvastatin caused a decrease in production of proinflammatory IL-1β in comparison to the effect of atorvastatin alone. PBMC incubated with aspirin and pravastatin jointly showed a decreased production of IL-2 and IFNγ and an increased generation of the anti-inflammatory cytokine IL-1ra.These findings are in agreement with other reports in the literature based on meta-analyses evolving clinical studies indicating that jointly administration of aspirin and statins results in decreased release of inflammatory cytokines and thus promote cardiovascular diseases prevention [28, 29]. Nakamura et al. [30] have observed improved inflammatory responses including cytokine down regulation in patients undergoing coronary artery bypass grafting 14 days after combined treatment with aspirin and atorvastatin.
5.Conclusion
The results of the present study underscore the importance of aspirin and statins in the immune activity of human peripheral blood mononuclear cells. Moreover, the findings contribute to the concept that combined administration of aspirin and statins, either hydrophobic or hydrophilic – atorvastatin and pravastatin respectively in our case, bears advantage in control of inflammation compared to the effect of each one of the drugs used alone.
References
- Wick J.Y.,Aspirin: a history, a love story,Consult. Pharm. 27:322-9. (2012).
- Ugurlucan M., Caglar I.M ., Cag lar F.N., Ziyade S., Karatepe O., Yild iz Y., Zencirci E., Ugurlucan FG., Arslan AH., Korkmaz S., Filizcan U., CocekS., Aspirin: fro m a h istorical perspective,Recent Pat.Cardiovasc. Drug Discov.7:71-6. (2012).
- Crusz S.M., Balkwill F.R., Inflammation and cancer: advances and new agents, Nat Rev Clin.Oncol.12:584-96 (2015).
- Grancher A., Michel P., Di Fiore F., Sefrioui D., Aspirin and colorectal cancer, Bull.Cancer.105:171-80(2018).
- Bousserouel S., Gosse F., Bouhadjar M., So ler L., Marescaux J., Rau l F., Long-term administration of aspirin inhibits tumour formation and triggers anti-neoplastic mo lecular changes in a pre-clin ical model of colon carcinogenesis,Oncol.Rep.23:511-7(2010).
- Berg man M., Djaldetti M ., Salman H., Bessler H., In flammation and colo rectal cancer: does aspirin affect the interaction between cancer and immune cells?, Inflammation.34:22-8(2011).
- Hussain M., Javeed A., Ashraf M., Zhao Y., Mukhtar M.M., Reh man M .U., Aspirin and immune system,Int.Immunopharmacol. 12:10-20(2012).
- Pant S., Patel N.J., Deshmu kh A., Go lwala H., Patel N., Badheka A., Hirsch GA., Mehta JL.,Trends in infective endocarditis incidence, microbio logy, and valve replacement in the United States from 2000 to 2011, J. A m. Coll.Cardiol. 19; 65:2070-6(2015).
- Ru more M.M., Aron S.M., Hiross E.J.,A review of mechanism of action of aspirin and its potential as an immuno modulating agent,Med. Hypotheses.;22:387-400(1987).
- Cesario T.C., Yousefi S., Carandang G.,The regulation of interferon production by aspirin, other inhibitors of the cyclooxygenase pathway and agents influencing calciu m channel flu x, Bull. N. Y. Acad. Med.65:26-35(1989).
- Endo A., A historical perspective on the discovery of statins, Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci.86:484-93(2010).Rev iew
- Quist-Paulsen P. Statins and inflammat ion: an update, Curr. Opin. Cardiol.25:399-405(2010.
- Bessler H., Salman H., Berg man M ., Straussberg R., Djaldetti M., In vitro effect of statins on cytokine production and mitogen response of human peripheral blood mononuclear cells, Clin. Immunol.117:73-7(2005).
- Berg man M., Salman H., Djaldetti M ., Bessler H., Stat ins as modulators of colon cancer cells induced cytokine secretion by human PBM C, Vascul. Pharmacol.5488-92(2011).
- Salman H., Berg man M., Djaldetti M ., Bessler H., Hydrophobic but not hydrophilic statins enhance phagocytosis and decrease apoptosis of human peripheral b lood cells in v itro, Bio med. Pharmacother.62:41-5(2008).
- Lopez-Pedrera C., Ruiz-Limon P., Valverde-Estepa A., Barbarroja N., Rodriguez-Ariza A., To cardiovascular disease and beyond: new therapeutic perspectives of statins in autoimmune d iseases and cancer, Curr. Drug Targets.13:829-41(2012).
- Kiener P.A., Davis P.M., Murray .JL., Youssef S, Rankin B.M., Ko wala M., Stimu lation of inflammatory responses in vitro and in vivo by lipophilic HM G-CoA reductase inhibitors, Int. Immunopharmacol.1:105-18(2001).
- Yilmaz A., Reiss C., Weng A., Cicha I., Stu mpf C., Stein kasserer A., Daniel W G., Garlichs CD., Differential effects of statins on relevant functions of human monocyte-derived dendritic cells,J. Leukoc. Biol.79:529-38(2006).
- Zhang O., Zhang J., Atorvastatin promotes human monocyte differentiation toward alternative M2 macrophages through p38 mitogen-activated protein kinase-dependent peroxisome proliferator-activated receptor γ activation, Int. Immunopharmacol. 26:58-64(2015).
- Lee S., Lee Y., Kim J., An J., Kim K., Lee H., Kong H., Song W., Kim K., Atorvastatin and rosuvastatin imp rove physiological parameters and alleviate immune dysfunction in metabolic disorders, Biochem. Biophys. Res. Co mmun. 478:1242-7(2016).
- Grip O., Janciauskiene S., Lindgren S., Atorvastatin activates PPAR-gamma and attenuates the inflammatory response in human monocytes, Inflamm. Res.51:58-62(2002).
- Crisby M., Modulation of the inflammatory process by statins,Timely Top. Med. Cardiovasc. Dis.9:E3(2005).
- Grip O., Janciauskiene S., Lindgren S., Pravastatin down-regulates inflammatory med iators in human monocytes in vitro, Eur. J. Pharmacol.410:83-92(2000).
- Takahashi H.K., Mori S., Iwagaki H., Yoshino T., Tanaka N., Weit z-Sch midt G., Nishibori M ., Differential effect of LFA 703, pravastatin, and fluvastatin on production of IL-18 and expression of ICAM-1 and CD40 in human monocytes.J. Leukoc. Biol.77:400-7(2005).
- Bespalova I.D, Riazantseva N.V., Kaliu zhin V.V., Murashev BIu., Osikhov I.A., Mediantsev IuA., Effect of atorvastatin on pro - inflammatory status (in vivo and in v itro) in patients with essential hypertension and metabolic syndrome, Kard iologiia.54:37-43(2014).
- Osnes L.T., Foss K.B., Joø G.B., Okkenhaug C., Westvik A.B., Ovstebø R., Kierulf P.,Acetylsalicylic acid and sodium salicylate inhibit LPS-induced NF-kappa B/c -Rel nuclear translocation, and synthesis of tissue factor (TF) and tu mor necrosis factor alfa (TNF - alpha) in hu man monocytes, Thromb. Haemost.76:970-6(1996).
- Hsia J., Sarin N., Oliver J.H., Go ldstein A.L., Aspirin and thymos in increase interleukin-2 and interferon-gamma production by human peripheral blood ly mphocytes,Immunopharmacology.17:167-73(1989).
- Chap man M.J., Fro m pathophysiology to targeted therapy for atherothrombosis: a role for the co mbination of statin and aspirin in secondary prevention, Pharmacol.Ther. 113:184-96(2007).
- Hennekens C.,Pravastatin and acetylsalycilic acid fixed-co mbination: a strategy to improve cardiovascular outcomes, Am J Card iovasc Drugs.7 Spec No 1:9-11(2007).
- Nakamu ra K., Masuda H., Kariyazono H., Arima J., Iguro Y., Yamada K., Sakata R., Effects of atorvastatin and aspirin co mb ined therapy on inflammatory responses in patients undergoing coronary artery grafting,Cytokine.36:201-10(2006).