Information
Journal Policies
An Autoimmune Hemolytic Anemia as the Initial Presentation of Acute Myeloid Leukemia
Jirawadee Noiwattanakul1, Juree Boondumrongsagoon1, Somchai Insiripong2*
2.Department of Medicine, Saint Mary Hospital, Nakhon Ratchasima 30000, Thailand
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction: Although an autoimmune hemolytic anemia can be occasionally found in some malignancies, especially chronic lymphocytic leukemia, it is still hardly reported in acute myeloid leukemia.
Objective: To describe an autoimmune hemolytic anemia in a Thai woman who was subsequently found to have acute myeloid leukemia.
Case Presentation: A 44-year-old Thai woman complained of gradual fatigue due to anemia for a month. She had no fever, no blood loss. The physical examination found only marked anemia without hepatosplenomegaly. Her blood tests included: Hb5.7 g%, WBC 7,190/mm3, platelet 160,000/mm3, blast 2 %, promyelocyte1 %, metamyelocyte2 %, band 1 %, N 74 %, L 17 %. The directanti-globulin test was found weakly positive butanti-ds DNA was negative. Her diagnosis was an autoimmune hemolytic anemia and she was treated with prednisolone 60 mg a day. Three weeks later, her anemic symptom was not improved, her blood was tested again, Hb 5.0 g%, WBC 171,000/mm3, platelet 174,000/mm3, blast 17 %, promyelocyte 16 %, myelocyte 6 %, metamyelocyte 5 %, band 4 %. Her bone marrow biopsy was shown to be acute myeloid leukemia. She could achieve complete remission after the treatment with idarubicin and cytosine arabinoside.
Conclusion: When the patients with an autoimmune hemolytic anemia were encountered, regular follow-up after treatment for finding out any associated disease should be considered. Otherwise the patient may not have received the appropriate diagnosis and treatment.
Autoimmune hemolytic anemia, Acute myeloid leukemia,Hematology
1. Introduction
An autoimmune hemolytic anemia (AIHA) is an acquired hemolytic disease due to the auto-antibody against the antigen presenting upon the surface of the own red blood cells. Besides the features of hemolysis, it is characterized by the positive direct anti-globulin test. AIHA may be classified into primary or idiopathic if it occurs spontaneously and secondary if it is found in some associated instances such as an autoimmune disease, malignancies, infections or drug usage [1-3].
In malignancies, AIHA could be occasionally seen and majority of them are found associated with chronic lymphocytic leukemia, teratoma [4], and other solid tumors such as renal cell carcinoma, and Kaposi’s sarcoma. AIHA can occur prior to, concurrently with cancers or after the end of treatment of cancers [5]. So far it has been hardly found in cases with myeloid leukemia, either chronic myeloid leukemia [6,7] or acute myeloid leukemia [8,9]. The aim of this report was to describe one case of AIHA that was diagnosed in a Thai woman who was later proved to have acute myeloid leukemia.
2. Case Presentation
A 44-year-old Thai woman developed gradual onset of fatigue due to anemia for a month without fever or frank blood loss. The physical examination revealed only frank pallor, no hepato-splenomegaly, no bruise.
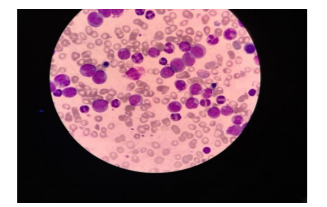
Her first blood test: Hb 5.7 g%, Hct 17.6 %, WBC 7,190/mm3, platelet 160,000/mm3, MCV 88.9 fl, MCH 28.8 pg, MCHC 32.4 g%, RDW 14.6 %, blast 1 %, promyelocyte 1 %, metamyelocyte 2 %, band 1%, Nucleated RBC 1/100 WBC, N 74 %, L 17 %, Hb analysis using the capillary zone electrophoresis method:HbA 96.5 %, HbA2 2.1 %, Hb F 0.6 %, Hb Constant spring (CS) 0.8 %,osmotic fragility test – negative, creatinine 0.75 mg%, BUN 11.9 mg%, direct anti-globulin test-weakly positive, indirect anti-globulin test-negative, anti-double stranded DNA-negative. The urinalysis was unremarkable. Her diagnoses were autoimmune hemolytic anemia (AIHA) and Hb Constant spring heterozygosity; she was treated with prednisolone 60 mg a day every day. Three weeks later, her anemic symptom did not improve and her blood test was tested again, Hb5.0 g%, Hct15.6 %, WBC 171,690/mm3, platelet 174,000/mm3, blast 17 %, promyelocyte 16 %, myelocyte 6 %, metamyelocyte 5 %, band 4 %, N 40 %, L 10 %, nucleated RBC 3 %, MCV 92.9 fl, MCH 29.8 pg, MCHC 32.1 g%, RDW 19.2 %. The direct anti-globulin test was negative. The bone marrow aspiration was not successful while the biopsy showed acute myeloid leukemia, marked with MPO, did not mark with CD34, TDT, CD117, CD3, and PAX- 5. Flow cytometric analysis of the bone marrow: CD13 (+), CD15 (+), CD11b (+), CD33 (+), CD64 (+), MPO (+) suggestive of acute myeloblastic leukemia with maturation AML-M2. Chromosome analysis appeared normal female pattern.
Her final diagnosis was acute myeloid leukemia. With the combination of idarubicin and cytosine arabinoside therapy, she could achieve complete remission within one cycle approved by the flow cytometric analysis and the direct anti-globulin test was never positive again. Then she successfully underwent to consolidation phase chemotherapy with high dose cytosine arabinoside.
3. Discussion
The diagnosis of AIHA in this patient solely depended on the positive direct anti-globulin test in the normochromic normocytic anemia patient with nucleated red blood cells in the peripheral blood [3].Likewise, the diagnosis of acute leukemia was based on the pathological finding of numerous blasts more than 20 % in the bone marrow and proved to be myeloid series by CD13, CD33 and MPO positive on the flow cytometry [10].
Actually corticosteroid is effective around 70-80 % in AIHA [11].The direct anti-globulin test in our case became negative after three-week prednisolone therapy and still negative when the patient achieved complete remission after induction of chemotherapy in spite of no corticosteroid or other immunosuppressant maintenance therapy. It seems closely similar to secondary AIHA associated with the solid tumor that is usually in remission after the tumor is curatively resected [5].
Because of the rarity, the pathogenesis of the emergence of AIHA in association with AML is not exactly studied [9]. The broken immunologic tolerance to the red blood cell antigen is proposed to be a major underlying mechanism leading to the emergence of AIHA [12].The CD4 (+)/CD25 (+) regulatory T cells may play a key role in this mechanism. Other mechanisms may include the molecular mimicry between the self-antigen and the foreign antigen, the polyclonal lymphocyte activation leading to failure to maintain self-tolerance [13].
4. Conclusion
A 44-year-old Thai woman was found to have severe anemia due to an autoimmune hemolytic anemia and later acute myeloid leukemia. An autoimmune hemolytic anemia should be considered as one contributing factor of severe anemia in a patient with acute myeloid leukemia.
References
- Valent P, Lechner K. Diagnosis and treatment of autoimmune haemolyticanaemias in adults: a clinical review. Wien KlinWochenschr 2008; 120(5-6): 136-51.
- Zeerleder S. Autoimmune haemolyticanaemia – a practical guide to cope with a diagnostic and therapeutic challenge.Neth J Med 2011; 69(4): 177-84.
- Park SH. Diagnosis and treatment of autoimmune hemolytic anemia: classic approach and recent advances. Blood Res 2016; 51(2): 69-71.
- Korubo KI, Nwauche CA, Ejele OA.Autoimmune hemolytic anemia in cancer patients at a tertiary center in Southern Nigeria. Blood 2013; 122(21): 4658. www.bloodjournal.org/content/122/21/4658
- Puthenparambil J, Lechner K, Kornek G. Autoimmune hemolytic anemia as a paraneoplastic phenomenon in solid tumors: A critical analysis of 52 cases reported in the literature. Wien KlinWochenschr 2010; 122(7-8): 229-36.
- Koksal A, Ozatli D, Haznedaroglu IC, Sunguroglu A, Karakus S, Buyukasik Y, Autoimmune hemolytic anemia in Philadelphia positive chronic myeloid leukemiawith t(7;14) anomaly after 5 years of interferon alpha treatment. Haematologia (Budap) 2002; 32(2): 163-7.
- Arbaje YM, Beltran G. Chronic myeloid leukemia complicated by autoimmune hemolytic anemia. Am J Med 1990; 88(2): 197-9.
- Tamura H, Ogata K, Yokose N, An E, Kamikubo K, Dan K, Kajii E, Nomura T. Autoimmune hemolytic anemia in patients with de novo acute myelocytic leukemia. Ann Hematol 1996; 72(1): 45-7.
- Essa N, El-Ashwah S, Denewer M, Essami Y, Mabed M. Autoimmune hemolytic anemia in a patient with acute myelomonocytic leukemia. J Blood DisordTransfus 2016; 7: 336. DOI: 10.4177/2155-9864.1000336.
- Estey EH. Acute myeloid leukemia: 2013 update on risk stratification and management. Am J Hematol 2013; 88(4): 318-27.
- Zanella A, Barcellini W. Treatment of autoimmune hemolytic anemia. Haematologica 2014; 99(10): 1547-54.
- Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev 2010; 24(3): 195-210.
- BarcelliniW. New insights in the pathogenesis of autoimmune hemolytic anemia. Transfus Med Hemother 2015; 42(5): 287-93.