Information
Journal Policies
Diagnosis of HCV Infection in Renal Chronic Infection Patients by Using ELSA and RT- PCR in Tikrit City
Hala Mohammed Majeed
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction: Viral hepatitis infection is an important cause of mortality and morbidity among patients treated hemodialysis and the spread of this disease varies from one region to another in the world. Chronic renal failure is the most common type of hepatitis C infection due to the need for blood transfusion and use of dialysis devices.
Methods: The study was conducted in the laboratories of the liver and digestive system. Hospital and in the city of Baghdad for the period from 1/1/2017 to 1/1/2018, which included the diagnosis of infection with viral hepatitis C of serum patients using the technique of Elisa and RT-PCR for patients who are deceased and incoming to Tikrit Hospital and clinics In the study, 100 samples were taken from 50 patients with renal failure and 50 healthy people aged 50-60 years, with clinical signs and according to the doctor's diagnosis, 5 ml of blood and serum were withdrawn into two parts (1 ml) For the purpose of isolating DNA and detecting the virus with RT-PCR technology and the second to purpose virus detection and enzyme Liver and kidney function using ELISA technique, SPSS version 16 was used in data analysis. The results were considered statistically significant at the level of P ≥ 0.05.
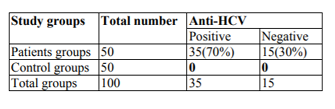
Results: The study result appearance of the 50 people with chronic renal failure (CRF), 35(70%) had positive an anti-HCV, while 15 (30%) had negative an anti-HCV antigen. While the results of renal function and liver enzymes showed a significant difference at P≥ 0.05 level and between infection with the C virus except for the glutamic–pyruvic transaminase (GPT) enzyme did not show a significant difference at the level P≥ 0.05, and the results also showed relationship between age and infection with the virus, which affects most age groups between 40-50 Years. HCV-RNA levels were also determined in positive and negative serum samples for ELISA test using Real Time-PCR was positive for 2.9% (1) positive Hepatitis C virus patients via Real-time-PCR as well as negative from enzyme-linked immunosorbent assay, whilst 28.6% (10) are positive HCV via enzyme-linked immunosorbent assay, but Not via Polymerase chain reaction.
Abbreviations: CRF=chronic renal failure, GPT=glutamic–pyruvic transaminase, HCV=Hepatitis C Virus.
HCV Infection, Renal Chronic infection, Patients, ELSA, RT- PCR,Hematology
1. Introduction
HCV disease is a main community health trouble with a global prevalence estimated at 3% HCV.Thereareabout180 million loaded and about Four million citizens a year are recently infected. (Flamm, 2013)
Hepatitis C virus is a 9.6-kb RNA virus that belongs to the virus (Flaviviridae) and Hepacivirus virus. Hepatitis C virus transmitted by blood, and includes known danger factor for spread of hepatitis C virus using injectable drugs, blood transfusion / blood produced. Implantation, chronic dialysis, working contact amongst health care staff, therapeutic injection, main / secondary surgery, dental cure, barber shops, unsafe sex, and vertical contact. (Yen et al., 2015 and Strader et al., 2004).
Patients with renal failure on dialysis at elevated risk for blood-borne infection due to long-term vascular contact and the possibility of contact to infection patients as well as polluted tools. disease as a result of HV are one of these infections, an chief cause of disease and death in dialysis People and a trouble in the organization of patients in renal dialysis unit. (Meyer’s etal., 2003) In India, a broad vary of hepatitis virus frequency rates (4.3% - 45.2%) in the dialysis population were Reported (Abacioglu etal., 2015 and Jasuja et al., 2009) There is a lack of available information of Hepatitis C Virus disease in Punjab. Since a great numeral of acute chronic renal failure patients call our tertiary care hospital, this retrospective learning be conduct decide positive antibody Hepatitis C Virus Abs in person undergo first-time transfusion at Guru Go bind Singh medicinal university, Hospital, Faridkot Punjab), its environmental plus region delivery.
In these patients, HCV disease is usually asymptomatic (Jaiswal etal., 2002) and be able to be diagnose by serological method as well as by amplification of HCV RNA (RT-PCR) (Yuki etal., 2000). Which differentiate among viraemic and non viraemic Hepatitis C Virus person in addition to use Hepatitis C Virus genotype (Young etal., 1993). HCV isolate have been categorize into six chief genotypes, numerous of which have a number of closely connected subtypes (Galan etal.,1998). New HCV variant from Vietnam, Jakarta and Indonesia have been explain as genotypes 7, 8, 9, 10 and 11(Simmond etal.,1994 and Tokita etal.,1994). The delivery of Hepatitis C Virus genotypes vary into diverse country when documented in blood donor, and haemodialysis and chronic hepatitis citizens (Tokita etal. 1996, Dusheiko etal. 1994 and Bosmansetal. 199
2. Methods
100 blood samples were collected (50 patients with renal failure and 50 healthy people aged 50-60 years, with clinical signs and according to the doctor's diagnosis from hemodialysis unit in Tikrit Hospital and clinics from (1/1/2017 to1/1/2018).
Blood was collected in the first hour before the blood-washing session. The samples were separated into two tubes for each patient, then frozen and stored immediately (-20 ° C) and -80°C respectively for viral and serological assays to reduce viral degradation. DNA, preventing mutual contamination is unnecessary. The third generation of Elisa Kits was used consistent with manufacturer instruction (ERBA Transasia, India). The group contains 100% sensitivity and specificity of ≥99% according to manufacturer, for the following signs: HCV IgG antibody and enzyme Liver and kidney function.
HCV RNA was extract from 200 μl of patient serum using sum viral DNA group according to the manufacturer instruction (Invitrogen, Carlsbad, CA, USA). The eluted genetic materials tore up at 70°C.
For HCV RNA discovery by RT-PCR, all serum samples were tested individually for the presence of HCV RNA by specific RT-PCR (Sacace Biotechnologies, REF V-1-100R, and Italy). To allow the molecular examination of a great amount of seronegative sample, the collected plan was developed, like the method describe previous (McOmish etal., 1994). This involves the synthesis of four negative serum samples and study of the combination because of HCV RNA. Twenty-five μl of every of the four samples were mix together, and then 100μl of the pond was use for the examine. The RT-PCR procedure is based on four main processes: isolation of HCV RNA from samples using RNA / DNA kit (Ribo-Sorb, Sacace Biotechnologies, REF K-2-1, Italy), reverse transcription of RNA using reverse primer, For M-MLV with the kit incubated in a cycler thermocouple at 37 ° C for 30 minutes, and then cDNA was amplified by PCR with special primers for the non-localized zone 5of the viral genome. Amplification was performed as follow: 95 ° C for 5 minutes, then 42 cycles of 95 ° C for 30 seconds, 67 ° C for 30 seconds, and 72 ° C for 30 seconds, follows via a finishing extension at 72 ° C for a minute one. After the products have been amplified on the agarose gel. The set contains internal control that can be used to conduct insulation and acts as an amplification control for each individual treated sample Determining potential inhibition of interaction. Negative and positive controls, inverse versions, were extracted and amplified in each batch of samples tested by polymerase chain reaction. Serum samples have been shown to contain HCV RV by RT-PCR. This ready-to-use PCR group contains basic materials, solutions, PCR main mix, positive control, negative control, molecular marker and loading dye (Figure.1). The procedure was done in accordance with manufacturers' instructions. Statistical examination was made using SPSS report 16 using crosstabs and Chi-square test. P-value ≥0.05 was considered as statistically significant.
3. Results
In the present study, a total number of 100 patients (50 patients with renal failure and 50 healthy people aged 50-60 years), are enroll for hemodialysis in the two following years (1/1/2017 to1/1/2018). Out of the sum50 patients, 24(48%) were establish to behave HCV infection. And the number of patients who give negative results for anti- HCV was 26(52%) Table1.
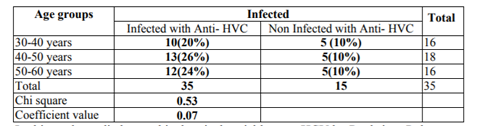
The maximum frequency was establishing in the 40-50 years of age group (26%) followed by 50- 60 years (24%) as well a slowly frequency was experimental in the age grouping30-40 years (20%) Table2.
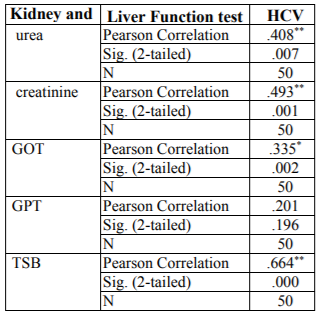
In this study studied some biochemical variables related to kidney function and liver enzymes. The results showed a significant raise (P < 0.05) in serum creatinine, GOT and TSB concentration and lessen significant (P < 0.05) in GPT concentrated in patients with chronic renal failure Table3.
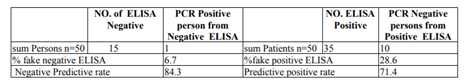
A significant variation was found among the study people and the two technique of the study. In the current study of a total of 50 patients, 35 (70%) of HD persons are positive Hepatitis C Virus, with ELISA. though, of the sum positive persons 2.9% (n=1) persons are positive for HCV by Real-time-Polymerase chain reaction in addition to negative of enzyme-linked immunosorbent assay, as 28.6% (10) were positive cases of HCV via enzyme-linked immunosorbent assay but not via Polymerase chain reaction. Privacy and sensitivity The third age group of ELISA compare to PCR over lapping RT for Hepatitis C virus was 97.8% and 80%, in that order. Positive analytical rate was 71.4% while the negative analytical rates 84.3% for enzyme-linked immunosorbent assay Table4. Hepatitis C disease has reach outbreak proportions worldwide and linked by numerous additional liver manifestations.
4. Discussion
Viral hepatitis is a major health problem worldwide, especially in tropical and subtropical regions. It is among the causes of morbidity and has already headed the list of infectious diseases reported in many countries. Chronic liver disease, especially due to HCV disease, is a main difficulty among patient with dialysis (HD) patients (Schneeberger etal., 1998). The high occurrence of HCV infection between patients treat with HD preservation has been credited to transfusion supplies in this group of risk (Chan etal., 1991). The frequency of viral hepatitis is larger in patients with HD than in general groups that affect value of life and death among patients. According to our results as shown in Table1, the total number of patients 100 (50 patients with renal failure and 50 healthy people aged 50-60 years) enrolled in this study; 35 (70%) were positive for hepatitis C virus and 15 (30) % of patients were negative for the hepatitis C virus. This finding was in agreement with the results of the study conducted by (Zeldis etal., 1991) to evaluate the hepatitis C virus in regular dialysis patients in BeniSuef between 70% of the study sample and a retrospective study conducted by (Senosy etal., 2016) on 186 patients in the HD unit in Casablanca, reported a high prevalence of HCV infection (76%) and the prevalence of HBV infection was reported at 2% (Boulaajaj etal., 2005). In contrast to the results of other studies, such as in Gaza, the prevalence of hepatitis B virus in patients with HD patients was 8.1%, 22% with HCV (Omaretal.,2003), in Basra - Iraq of 122 patients with HBV infection were positive (50%), While HCV seropositivity (42.6%) was 5 in Kosovo out of 583 HCV prevalence (12%) (El-Ottol etal,. 2010). In the case of Tocantins, Brazil of HCV antibodies was detected in 13% of patients (Shihab etal., 2014). In Amman, Jordan, the prevalence of HCV in HD patients was 5.9% (Telaku etal., 2005). The variance in the ratios in the different studies may be due to the difference in the size of the samples, the sensitivity of the methods and the specificity of the methods used in the detection of the anti-HCV Abs. In relation to age and type C infection among chronic renal failure patients, the results shown in Table.2 showed that with age, infection rate with HCV is increased (26%).this rate was establish in the age group of 40-60 years. The results were consistent with (Al Hijazat, and Ajlouni2008) in Jordan, who reported that the age factor had a significant effect on type C infection with respect to HCV infection, the current rate was low (26%) compared to the number of countries in the world, in Libya, Palestine, Jordan and Turkey 20.2% ,28%, 24%, 31.1% respectively, (Ghazzawi etal.,2015,Alasheketal.,2012 and Al. Jamal ,2009) While the results of the virus type C high compared to Recorded some studies of the doses in Sudan, Bahrain and Saudi Arabia with92%, 59%, 85% Respectively (Yakaryilmaz etal., 2006, Gasim, etal., 2012 and Reddy etal., 2005).
The results in Table.3 showed a significant increase (P >0.05) in the concentration of urea, creatinine, bilirubin, GOT and GPT. The same table showed a significant decrease (P < 0.05) in the GPT concentration among patients with dialysis Compared with the control group. These results were consistent with the studies conducted by (Qadi etal., 2004 Mehdi etal., 2012 and Merzah etal., 2015) in both Baghdad, Waist and Sudan respectively.
Both urea and creatinine are considered nitrogenic substances in the blood, and doctors depended on the concentration of these nitrogenous wastes to determine whether the patient has kidney disease, as these tests help determine the efficiency of kidneys in the clearance of blood from these wastes or toxins (Mehdi etal., 2012). Patients with chronic kidney failure are a dangerous factor in the collection of waste or nitrogenous toxins, so treatment of renal failure in the hemodialysis leads to rid the body of these uremic toxins, depending on the effect of the efficiency process of dialysis and fluid structure used in the process of dialysis (Merzah etal., 2015)
The significant increase in the concentration of urea, creatinine and bilirubin in the serum of chronic renal failure patients treated with hemodialysis in the present study compared to the control group may be due to the incomplete filtration of these substances by dialysis or due to the stimulation of the internal structure or deterioration during the dialysis session.
The researcher's study (Ahmed etal., 2016) confirmed that the high level of creatinine indicates a decrease in glomerular filtration rate thus reducing the efficiency of kidneys in detoxification.
The high efficiency of liver enzymes may be due to the effect of liver cell membranes and change their effectiveness and destruction, which leads to the disruption of the transfer of metabolites and the leakage of these enzymes into the bloodstream and high serum levels of patients, or may be due to oxidative stress and the formation of free radicals that lead to harmful structural and functional changes in the liver cell such as, Peroxide lipid in the cell membrane , GOT and GPT enzymes are present in both the liver and the kidney so any damage to the kidney or liver or their tissues results in an increase in these enzymes in patients' serum (Shahbazian etal., 2009 and Damera etal., 2011).
In this study, a few negative pesons were detected falsely by ELISA, using RT-PCR (1, 6.7%). This shows that PCR-based assay is capable to verify accurate amount of HCV RNA in serum, as previously report (Benoudaetal. 2009). PCR specifically help to resolve weak ELISA-positive results in the presence of clinical markers consistent with HCV infection and / or danger factor though, the outcome of the two methods must be interpret by care when the discovery of Hepatitis C Virus RNA typically precede discovery Abreaction in serum following acute introduction. Hepatitis C Virus RNA can be recognized as early as two weeks after introduction, while HCV Abs are not usually detect 8-12 weeks ago (Tashkandy etal., 2007). On the other hand, throughout the path of disease when virus is clean, just the antibodies Remain positive, as well as RNA are usually not detect. Thus, PCR discovery rate was lesser when ELISA was use as a gold normal (Umar, 2011). In this study, 70% of Hepatitis c virus persons are positive by both Real-time– Polymerase chain and enzyme-linked immunosorbent assay indicate that Hepatitis c virus disease are acute or chronic by scientific context. In 6.7% of Hepatitis c virus samples, the results are positive via Real-time– Polymerase chain and negative interlaced by Real Time – Polymerase chain enzyme-linked immunosorbent assay. This may point to an early on Hepatitis c virus disease, chronic Hepatitis c virus in patients with chronic immunodeficiency or HCV RNA positive false examination. PCR consequences were report as negative interleaved RT and positive in 10 (28.6%) which may indicate HCV solution, acute HCV during a low period, or anti-viral C. The kindliness and privacy of in enzyme-linked immunosorbent assay the present study are 80% and 97.8%, respectively; there are good sufficient to perform an analytic examination. equally, the specificity of RT-PCR was total at elevated kindliness (100%) noting to it was not just appropriate for experimental analysis but also appropriate for show to Hepatitis c virus disease stops pread of the sickness (Wang etal.,2004 and Ghanyetal., 2009).
5. Conclusion
ELISA tests have several advantages in investigative preparation include cease mechanization, case of utilize, relation rise efficiency, and low down changeability. Though, as with all immunohistochemistry assays, the false positive outcomes are sometimes a trouble through the third age group of enzyme-linked immunosorbent assay, or further or assertive. A test such as PCR overlapping RT is often useful. Additional studies are suggested to study the genetic patterns of Hepatitis C to help enhanced scientific outcome and epidemiological study and to supply in formation with significant implication for the scientific organization of hepatitis C and vaccine advance.
The ethical committee of the concerned institute approved the research protocol. The purpose and procedures of the study were to be explained to all the study subjects, and informed consent was to be obtained from them.
References
- Abacioglu YH, Bacaksiz F, BaharIH, and Simmonds P. Prevalence of anti-HCV and HCV viremia in hemodialysis patients Molecular. Am J Kidney Dis.2015; 42(5):631-57.
- Al Hijazat M, and AjlouniY. Hepatitis B Infection among Patients Receiving Chronic Hemodialysis at the Royal Medical Services in Jordan; Saudi J Kidney Dis Transpl 2008; 19(2): 260-267.
- Alashek WA, MclntyreCW, and Taal MW. (2012). Hepatitis B and C infection in haemodialysis patients in Libya: prevalence,incidence and risk factor. BMC Infectious Diseases .2012; 12(1): 1471-2334.
- Abu nwais JQ, and Idris OF. Prevalence of hepatitis C, hepatitis B and HIV infection among hemodialysis patients in Jenin District (Palestine). Iranian Journal of Virology.2010; vol 4(2), pp: 38-44.
- Al Jamal M, Al Qudah A, and Al Shishi K. Hepatitis C virus infection in hemodialysis patients in the south of Jordan. Saudi J Kidney Dis Transpl .2009; 20(1), pp: 488-92.
- AhmedME and Idres FI.Effect of Hemodialysis on serium uric acid, urea, creatinine and Albumin level in chronic renal failure patients. Pyrex Journal of Biomedical Research.2016; Vol 2(6), pp: 48-51.
- Bosmans JL, Nouwen EJ, Behets G et al. Prevalence and clinical expression of HCV-genotypes in haemodialysis patients of two geographically remote countries: Belgium and Saudi-Arabia. ClinNephrol 1997; 47: 256–262.
- BoulaajajK, ElomariY, ElmalikiB, MadkouriB, ZaidD etal. Prevalence of hepatitis C, hepatitis B and HIV infection among haemodialysis patients in Ibn-Rochd university hospital, Casablanca; NephrolTher. 2005; 1(5): 274-84.
- Benouda A, Boujdiya Z,Ahid S, Abougal R, and Adnaoui M. prevalence of hepatitis C virus infection in morocco and serological test assessment of detection for the viremia prediction. PatholBiol (Paris).2009; vol57 (5), pp: 368-72.
- Chan TM, ok AS. and Cheng IK. Hepatitis C infection among dialysis patients: a comparison between patients on maintenance haemodialysis and continuous ambulatory peritoneal dialysis.1991; Nephrol Dial Transpl; 6:944-7.
- Dusheiko G, Schmilovitz-Weiss H, and Brown D.et al., Hepatitis Cvirus genotypes: an investigation of type-specific differences in geographic origin and disease. Hepatology. 1994; 19(6): 13–18. - Damera S, Raphael KL, Baird BC, CheungAK, Greene T, and Beddhu S. Serum alkaline phosphatase level associate with elevated serum C-reactive protein in chronic kidney. Kidney Int. 2011; vol 79(2), pp: 228–233.
- El-Ottol A,Elmanama A, and AyeshB. Prevalence and risk factors of hepatitis B and C viruses among hemodialysis patients in Gaza strip, Palestine; Virology Journal.2010;7 (210): 1-7.
- Flamm SL, Hepatitis C. Initiative for vaccine research JAMA. 2010;289(7): 2413-2417
- Galan F,Perz-Gracia, MT,LozanonA, Benavides B, Fernandez- Ruiz E, and Rodriguez-Iglesias M.A. A 3-year follows up of HCVRNA viraemia in haemodialysis patients. Nephrol Dial Transplant1998; 13(7): 1211–1214.
- Ghazzawi I,Yasssin M,Alshebly H,Sheyyab S,Algudah B, and Alwahadni N. Prevalence of Hepatitis B and C viruses in Hemodialysis patients at JRMS. JRMS March2015;vol 22(2), pp: 69-75
- Gasim GI. Hamdan, H.Z.; Hamdan, S.Z. and Adam, I. (2012) Epidemiology of hepatitis B and hepatitis C virus infection among hemodialysis patients in Khartoum. J Med Virol.vol84 (1), pp: 52-5.
- Ghany, M.G.; Strader, D.; B.; Thomas, D.; L.;(2009). Steef LB. Diagnosis, management of treatment of hepatitis C: an update. Hepatology,; 49:1335–1374
- Jasuja, S.; Gupta, A.K.; Choudhary, R.; Kher,V.; Agarwal D.K,; and Misra, A.;(2009) Indian J Nephrol.19:62-68.
- Jaiswal, S.; Chitnis, D.; Salgia, P.; Sepaha. A.; andPandit. C. (2002) Dialysis Transplant.31:234-38.
- Meyers, C.M.; Seef, L.B.;Breen, C.O; Hoofangle, J.H.;(2003) Molecular evidence for nosocomial transmission of hepatitis C virus in a French hemodialysis unit. J Med Virol; 58: 139–144.
- McOmish, F.; Yap, P.L.; Dow, B.; C.; et al., (1994). Geographical distribution of hepatitis C virus genotypes in blood donors: an international collaborative study. J ClinMicrobiol 1994; 32: 884–892.
- Mehdi, W.A.; Al-Helfee, W.; A.; and Dawood, A.; S.; (2012). Study of several oxiodants, Total Acid phosphatase, Prostatic Acid phosphatase, total and free prostate-specific antigen in sera of man with chronic kidney failure. Kerbala J. Pharm. Sci.pp: 155-165.
- Merzah, K.S. and Hasson, S.F. (2015). The Biochemical Changes in Patients with Chronic Renal Failure. Int. J. Pharm. Med. Biol. Sci.vol 4(1), pp: 75-79.
- Omar, M.; N.; Tashkandy, M.';( 2003). A: Liver enzyme and protein electrophoretic pattern in hemodialysis patients with antibodies against the hepatitis C virus. Saudi Med J; 2:S122.
- Qadi, A.A.; Tamim, H.; Ameen, G.; Bu-Ali, A. ; Al-Arrayed, S. ; Fawaz, N.A. and Almawi, W.Y. ( 2004). Hepatitis B and Hepatitis C virus prevalence among dialysis patients in Bahrain and Saudi Arabia: A survey by scrologic and molecular methods. American Journal of Infection control. 32, pp: 493-495.
- Reddy, G.A.; Dakshinamurthy, K.V.; Neelaprasad, P.; Gangadhar, T. and Lakshmi, V. (2005). Prevalence of HBV and HCV dual infection in patients on haemodialysis. Indian Journal of medical Microbiology .23, pp: 41-43.
- Strader,D.;B.;Wright,T,;Thomas,D.L.;andSeeff, L.B.;(2004) Hepatology.39:1147-1171.
- Simmond, s. P.; Alberti, A.; and Alter, H.J.; et al., (1994) A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology; 19: 1321–1324.
- Schneeberger, P.; M. Keur, I.; Vliet, W.V.; et al. (1998). Hepatitis C Virus Infections in Dialysis Centers in the Netherlands: a National Survey by Serological and Molecular Methods. Journal of Clinical Microbiology. 36 (6):1711– 1715.
- Senosy, S.; A.; and El Shabrawy, E.; M. ;(2016). Hepatitis C virus in patients on regular hemodialysis in Beni-Suef Governorate, Egypt. J Egypt Public Health Assoc.; 91(2):86-9.
- Shihab, S.;Al-Hmudi, H.; Al-Edani, H.; and Mahdi, K.; (2014).Viral hepatitis infections in Basrah hemodialysis unit: Serological diagnosis and viral loading; European Journal of Experimental Biology, 4(2): 106-112.
- Shahbazian, H.; Moghadam, A.; Ehsanpour, A. and Khazaali, M. (2009). Changes acid before and after hemodialysis. Iran J Kidney Dis. vol 3(3), pp: 151-5.
- Tokita, H.; Okamoto, H.; and Tsuda, F.; et al., (1994). Hepatitis C virus variants from Vietnam are classifiable into the seventh, eighth and ninth major genetic groups. ProcNatlAcadSci USA; 91: 11022–11026.
- Tokita H.; Okamoto, H.; andIizuka, H.; et al., (1996). Hepatitis C virus variants from Jakarta, Indonesia classifiable into novel genotypes in the second (2e and 2f), tenth (10a) and eleventh (11a) genetic groups. J Gen Virol; 77: 293–301.
- Telaku, S.; Fejza, H.; Elezi, Y.; and Bicaj, T.; (2009). Hepatitis B and C in dialysis units in Kosova; Virology Journal; 2009, 6 (72): 1-4.
- Tashkandy; M.;A., Khodari YA., Ibrahim AM., Dhafar KO, Gazzaz ZJ, Azab, B. A.(2007) Evaluation of the available anti-HCV antibody detection tests and RT-PCR assay in the diagnosis of hepatitis C virus infection. Saudi J. Kidney Dis. Transpl,; 18:523–531.
- Umar, M.; Hamama-tul-Bushra, A.; Ahmad, M.; Khurram, M.; Usman, S.;Arif, M.;et al.,(2010).Hepatitis C in Pakistan: A Review of Available Data. Hepat Mon; 10(3):205-214
- Yen,T.; Keeffe, E. B.; and Ahmed, A.; (2015).Hepatitis C Virus infection in dialysis patients : A retrospective study from a tertiary care hospital of north india J Clin Gastroenterol36: 47-53
- Yuki, N.; Ishida, H.; and Inoue, T.; et al. (2000) Reappraisal of biochemical hepatitis C activity in hemodialysis patients. J ClinGastroenterol; 30: 187–194.
- Young, K.K.; Resnick, R.M.; and Myers, T.; W.;(1993). Detection of hepatitis C virus RNA by combined reverse transcription-polymerase chain reaction assay. J ClinMicrobiol 31: 882– 886
- Yakaryilmaz, F.; Gurbuz, O.A.; Guliter, S.; Mert, A.; Songur, Y. Karakan, T. and Keles, H. (2006). Prevalence of occult hepatitis B and hepatitis C virus infections in Turkish hemodialysis patients. Ren Fail. Vol 28(8) , pp: 729-735
- Zeldis, J.; B.; Depner, T.A.; Kuramoto, I.K.; et al., (1990). The prevalence of HCV antibodies among haemodialysis patients. Ann Intern Med; 112:958-60.
- Wang, N.; Gao, X.Q.; Han, J.X.;(2004). Simultaneous detection of HBV and HCV by multiplex PCR normalization. World J. Gastroenterol,; 10:2439–2443.