Information
Journal Policies
The Effect of Malaria Parasitaemia on some Full Blood Count Parameters of Pregnant Women in Sokoto, North Western Nigeria
Ibrahim A.B1,Erhabor O1,Udomah P.F1,Mairo H2,Yakubu A1,Bello M3,Ibrahim K.K1,Buhari H.A1,Abubakar S5,Mustapha U.K4
2.Department of Gynaecology, Usmanu Danfodiyo University Teaching Hospital, Sokoto.
3.Department of Medical Microbiology, Faculty of Medical Laboratory Sciences, Usmanu Danfodiyo University, Sokoto.
4.Department of Immunology, Faculty of Medical Laboratory Sciences, Usmanu Danfodiyo University, Sokoto.
5.Women and Children Hospital Idi Gombe, Gombe State.
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction:Malaria in pregnancy is one of the major causes of anemia, stillbirth, abortion and foetal death in sub-Saharan African countries. This comparative study was designed to determine the effect of malaria parasitaemia on some full blood count parameters among pregnant women attending antenatal clinic in Specialist Hospital Sokoto.
Materials and Methods:Fifty (50) blood samples of febrile pregnant women were collected and processed. Malaria testing for parasite identification was carried out with Real-time Polymerase Chain Reaction (qPCR) using Gene Finder Malaria Real Amp kit (INFOPIA Co., Ltd., Korea). Full Blood Count testing was carried out using Mythic 22 CT (Orphee, Switzerland).
Results: The pregnant women were within the age range of 18-42 years with mean age of 26.72±6.207 years. Out of the 50 blood samples of febrile pregnant women tested for malaria, 14 were positive for malaria parasite and a total of 28.0% prevalence of malaria. The overall prevalence of anemia in this study was 18(36.0%). MCHC was statistically significant between parasitized and non-parasitized pregnant women (p= 0.00). There were some differences in the mean ± SD of the remaining full blood count parameters but the differences were not statistically significant (p˃0.05).
Conclusion: The study showed that malaria has significant effects on the red blood cells parameters resulting to anemia. Full blood count should be routinely carried out for pregnant women attending antenatal clinics in order to diagnose and monitor the incidence of anemia.
1. Introduction
Malaria is a major public health problem especially in tropical and sub-tropical areas. It is estimated to be responsible for about 1 to 3 million deaths and 300-500 million clinical cases annually[1],[2]. Malaria is a tropical disease caused by a parasite that is transmitted from human to human by the female Anopheles mosquito. The mosquito carries the parasites plasmodium and transfers it to the host through a bite. Plasmodium infection can result in serious complications and conditions such as maternal anemia, fever, foetal anemia, abortion, still-birth, and even death of the child or mother before birth or soon after delivery. Malaria is a preventable and treatable disease. If malaria is diagnosed and treated early, the duration of the infection can be considerably shortened, which in turn reduces the risk of complications and death[3]. The vast majority of cases are children under the age of five years and pregnant women[4].
Changes in hematological parameters are likely to be influenced by any disease condition including endemic diseases, such as malaria, that can affects health of mankind with various clinical presentations. Hematological changes are some of the most common complications in malaria and they play a major role in malaria pathogenesis. These changes involve the major cell types such as red blood cells (RBCs), leucocytes and thrombocytes[5].
The pathophysiology of malaria results from destruction of erythrocytes with the liberation of parasites and erythrocyte material into the circulation and host reaction to these events. P. falciparum malaria infected RBC’s also sequester in the microcirculation of vital organs interfering with microcirculatory flow and host tissue metabolism [6]. A glycolipid material is released from the rupture of schizont. This parasite product induces activation of the cytokine cascade. Cells of the macrophage monocyte series and endothelium are stimulated to release cytokines. Initially tumour necrosis factor (TNF) and interleikin -1 (IL-1) are produced and these in turn induces the release of other cytokines including IL-6 and IL-8. These cytokines are responsible for many of the symptoms and signs of the infection, particularly fever and malaria[6].
Red cells Sequestration is a process whereby erythrocytes containing mature forms of P. falciparum adhere to microvascular endothelium (cytoadherence) and this disappear from the circulation. Sequestration occurs predominantly in the venules of the vital organs. It is not distributed uniformly throughout the body being greatest in the brain, particularly the white matter, prominent in the heart, liver, kidney, intestines and adipose tissue and least in the skin. Cytoadherence and the related phenol-menon of rosetting lead to microcirculatory obstruction and reduced oxygen and substrate supply leading to anaerobic glycolysis and lactic acidosis6. Cytoadherence is mediated by a family of strain specific high molecular weight parasite derived proteins termed P. falciparum erythrocyte membrane protein -1 (EMP -1) or PF, EMP -1. This protein is exposed to the surface of infected erythrocyte. These proteins are present as humps or knobs on the surface of red cells and these are point of attachment to vascular endothelium. Several different sticky proteins present on the surface of vascular endothelium have been shown to bind parasitized red cells. The most important of these proteins is the leucocyte differentiation antigen CD36. Other proteins are intracellular adhesion molecule -1 (ICAM -1).Vascular cell adhesion molecule (VCAM -1) and endothelial leukocyte adhesion molecule -1 (ELAM -1).
ICAM -1 appears to be the major ligand in the brain involved in cerebral sequestration and CD36 is probably the major ligand in other organs[6]. Erythrocytes containing mature parasites also adhere to uninfected RBC’s. It mainly occurs at the middle of the asexual cycle. Rosetting might encourage cytoadherence by reducing flow which will enhance available glycolysis, reduce pH and facilitate adherence of infected erythrocytes to venular endothelium[6]. As the parasite matures inside the erythrocytes the normally flexible biconcave disc becomes progressively more spherical and rigid infected red cell are less filterable than uninfected cells6. Normochromic and normocytic anemia is a common and frequently severe complication of malaria, particularly in young children and pregnant women. In some endemic areas it can account for more than 50% of malaria-associated mortality. The pathogenesis of severe anaemia (defined as Hb < 5g/dL) during malaria infection is not fully defined[7]. Both increased destruction of infected red cells and decreased production of red cells in response to anaemia due to dyserythropoiesis appear to play a role. There is very good evidence of accelerated destruction of uninfected red cells but the mechanism by which uninfected red blood cells are destroyed has not been elucidated. Marked splenomegaly during acute infection reflects extensive sequestration of red cells by the spleen resulting in anemia[8].
2. Materials And Methods
This study was carried out in Specialist Hospital Sokoto, North-Western Nigeria. Sokoto is the capital city of Sokoto State, Nigeria. The State is located between longitude 11’30°, 13’50° East and latitude 4’to 6’0° North. It is bordered to the North by Niger Republic, Zamfara State to the East and Kebbi State to the South-West[9]. The State falls within the Savannah vegetation zone. Rainfall starts late and ends early with mean annual rainfall ranging from 500 to 1,300 mm. The State has three peculiar seasons; the cold dry (November-February), hot dry (March-July) and wet. The wet season begins in most part of the State in May and lasts up to September.
The State is made up of Hausa and Fulani as the majority and a minority of Zabarmawa and Tuareg and o ther non- indigenous settlers. The two major languages in the State are Hausa and Fulfulde (spoken among the Fulani). The main occupation of the people is grain production and animal husbandry. Majority of the indigenous people practice agriculture. Crops produced include; commercial crops like millet, sorghum, beans, rice and maize. Other occupations commonly practiced are dying, blacksmithing, weaving, carving, trading, and cobbling. Sokoto ranks second in livestock production in Nigeria. Modern Sokoto city is a major commercial centre in leather crafts and agricultural products. Occupation of city inhabitants also include trading, commerce, with a reasonable proportion of the population working in private and public sectors. The vast Fadama land of the Sokoto-Rima River system dissects the plain and provides rich alluvial soil and extensive grassland fit for a variety of crop cultivation, hence farming and livestock rearing are the principal activities in the State[10]. Based on 2006 population census, Sokoto State had a population of 3,696,999 with a projected population of 4,695,188.8 in 2015 based on the population annual growth rate of 3%[11].
Subjects for this study included consecutively-recruited pregnant women attending antenatal clinic in Sokoto Specialist Hospital. This cross sectional comparative study was designed on fifty (50) febrile pregnant women attending antenatal Clinic of the Specialist Hospital, Sokoto. Inclusion criteria include febrile pregnant women, age ≥ 18 years, confirmation of pregnancy by a consultant obstetrician and willingness to offer a written informed consent to participate in the study. Non- pregnant women, non-febrile pregnant women, pregnant women < 18 years and non -consenting pregnant women were excluded from the study. Ethical clearance was obtained from the Ethics and Research Committee of Sokoto State Specialist Hospital, Sokoto and written informed consent was obtained from all participants in the study.
Three milliliters (3ml) of blood was collected by venepuncture using aseptic technique from each participant and introduced into Ethylene Diamine Tetra acetic Acid (EDTA) anticoagulant tube. Each sample was then mixed gently and thoroughly to ensure anticoagulation. The samples were analyzed for malaria testing and determination of full blood count (FBC) parameters. Diagnosis of malaria using Real-time PCR (Gene Finder Malaria Real Amp kit) with International Standard Organization (ISO) number, ISO 9001; 13485 manufactured by INFOPIA Co, Ltd. (Korea). Mythic 22 CT a fully automated 5 part-differential hematology analyzers was used for analyzing the Hb, WBC total and differential leucocyte counts and platelet count (Orphee, Switzerland).
The data obtained was analyzed using SPSS version 20 (SPSS Inc., Chicago, IL, USA, 2011). The results were expressed as percentage and Mean ±SD. Comparison was made using analysis of variance (ANOVA), paired comparison was carried out using the student t-test and p-value of equal to or less than 0.05 (p ≤0.05) was considered significant.
3. Results
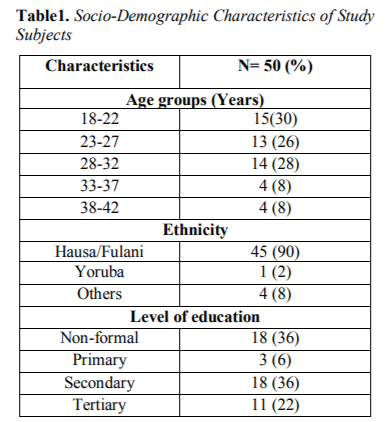
This study included, 50 consecutively-recruited pregnant women presenting to the antennal care in Specialist Hospital Sokoto with history of febrile illness. The age range of the subjects was 18-42 years with mean and standard deviation of 26.72±6.207 years. Out of 50 febrile pregnant women screened for malaria, 14 were positive using PCR technique with a total of 28.0% prevalence of malaria. Table 1 showed the Socio-Demographic characteristics of study subjects.
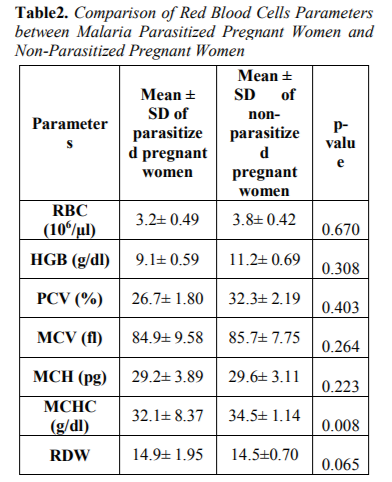
Table 2 showed the Comparison of Red blood cells parameters between malaria parasitized pregnant women and non-parasitized pregnant women. Mean cell haemoglobin concentration (MCHC) parameter was statistically significant between malaria parasitzed and non-parasitized subjects. The remaining red blood cells parame - ters; Red blood cells count (RBC), Parked cell volume (PCV), Mean haemoglobin (HB),Mean cell volume (MCV), Mean cell haemoglobin (MCH) and Red cell distribution width (RDW) were reduced between malaria parasitized and non-parasitized pregnant women but not statistically significant (p> 0.05).
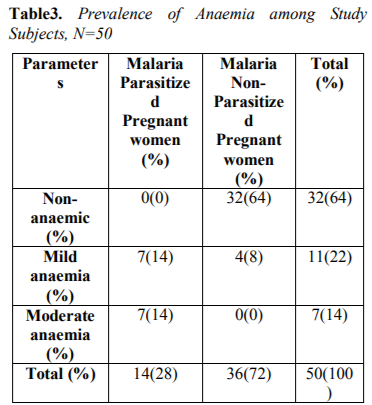
Table 3 showed Prevalence of anemia among study subjects. The overall prevalence of anaemia observed in this study was 18(36%), out of which 7(14%) had mild anaemia, 7(14%) had moderate anaemia among malaria infected pregnant women compared to 4(8%) had mild anaemia among malaria non-infected pregnant women.
4. Discussion
Anaemia in pregnancy was defined as Packed Cell Volume (PCV) less than 30% or haemoglobin less than 10.0g/dl[12]. The degrees of anaemia were classified as mild (PCV 27-29%), moderate (PCV 19-26%) and severe (PCV below 19%).
The overall prevalence of anaemia observed in this study was 18(36%), out of which 7(14%) had mild anaemia, 7(14%) had moderate anaemia among malaria infected pregnant women compared to 4(8%) had mild anaemia among malaria non-infected pregnant women. This report was at variance with the report of Udomah et al[13] who reported the proportion of subjects with anaemia among the malaria parasitized patients was 21.88% compared to 12.37% among the non-parasitized patients. This study shows that the haemoglobin, PCV and RBC were reduced in parasitized pregnant women (9.1±0.59,26.7±1.80and3.2±0.49) compared to non-parasitized pregnant women (11.2± 0.69, 32.3± 2.19 and3.8± 0.42) respectively. The differences were however not statistically significance difference. This finding is similar with previous report of Erhabor et al14 but at variance with report of Lee et al[15]. The reduced MCHC observed in this study among malaria parasitized pregnant women compared to non-parasitized pregnant women was at variance with the report of Adesina et al[16] indicating increased MCHC among parasitized pregnant women when compared with non-parasitized pregnant women. The reduced PCV observed in this study is in accordance with the report of Abudu[10]. indicating moderate anaemia among parasitized pregnant women. The pathogenesis of anaemia in malaria is particularly complex, multi factorial and incompletely understood. It is thought to result from a combination of haemolysis of parasitized red blood cells; accelerated removal of both parasitized and innocently un-parasitized red blood cell, depressed as well as ineffective erythropoiesis with dys-erythropoietic changes and anaemia of chronic disease. Other possible causative factors for anaemia in malaria include; decreased red blood cell deformability, splenic phagocytosis and or pooling and increased rate of clearance from the circulation. Tumour necrosis factor alpha (TNF-α) has also been implicated and may cause ineffective erythropoiesis. Anaemia develops because of direct parasitization of erythrocytes by plasmodium resulting in lysis of infected cells[17].
5. Conclusion
In conclusion, the study showed moderate prevalence of malaria despite the fact that the research was carried out during raining season, malaria has significant effects on the red blood cells resulting to moderate anemia among the study subjects.
Recommendation
We recommended pregnant women presenting with febrile illnesses should be screened for malaria and Full blood count should be routinely carried out for pregnant women in order to diagnose and monitor the incidence of anaemia
References
- Adegnika, A.A., Ramharter, M., Agnandji, S.T., Ateba Ngoa, U., Issifou, S., Yazdanbahksh, M. and Kremsner, P.G. Epidemiology of parasitic co-infections during pregnancy in Lambaréné, Gabon. Trop Medicine Inter. 2010; 15 (10): 1204-1209.
- Wahinuddin, S. and Brian, C.M.K. Typhoid and malaria co – infection – An Interesting finding in the investigation of a tropical fever.Malay J of Med Sci. 2006; 13(2): 64-65.
- Hall, N., Marianna, K., Raine, A. A. (2005). Comprehensive Survey of the Plasmodium Life Cycle by Genomic, Transcriptomic, and Proteomic Analyses. J of Orient Sci Res. 2005;307(5706):82-86.
- Olasehinde, G.I., Yah, C.S., Singh, R., Olusola, O., Ajayi, A.A., Valecha, N., Abolaji, A.O.,Adeyeba, O.A. Genetic Diversity of Plasmodium falciparum field isolates from south Western Nigeria. Afr Heal Sci. 2012; 12(3):355- 361.
- Bakhubaira, S. Hematological parameters in severe complicated Plasmodium falciparum malaria among adults in Aden. Turk J of Haematol. 2013; 30:394–399.
- White, N.J. Malaria Mansons Tropical Disease. 12th Edition. Elsevier, Canada. 1996: 1087– 1151.
- Haldar K, Mohandas N. Malaria, erythrocytic infection, and anemia. Hematology Am Soc Hematol Educ Program. 2009:87–93.
- Safeukui I, Correas JM, Brousse V, Hirt D, Deplaine G, Mule S, Lesurtel M, Goasguen N, Sauvanet A, Couvelard A, Kerneis S, Khun H, Vigan-Womas I, Ottone C, Molina TJ, Treluyer JM, Mercereau-Puijalon O, Milon G, David PH, Buffet PA. Retention of Plasmodium falciparum ring-infected erythrocytes in the slow, open microcirculation of the human spleen. Blood. 2008; 112:2520–2528.
- Mamman, A.B. Sokoto State. In: A People United, a Future Assured (Survey of States). Mamman, A.B., J.O. Oyebanji and S.W. Peters (Eds.). Vol. 2, Gabumo Publication Co. Ltd., Calabar, Nigeria. 2000: 123-134.
- Sokoto State Diary. Ministry of Information. Sokoto, Sokoto State. 2003: 4-5.
- National Population Commission. National Census Figures, Abuja, Nigeria. 2006: 206-210.
- Abudu, O.O. Anaemia in pregnancy. In: Agboola, A., editor. Text book of Obstetrics and Gynaecology for Medical Student, Nigeria. Heinemann. 2001; 11: 77-89.
- Udomah, F., Zama, I., Abdulrahaman, Y., Asemota, E., E menike, F. Anaemia and Parasite Density in Malaria Infected Patients in University Of Calabar Teaching Hospital, Calabar, Nigeria. Sokoto J of Med Lab Sci. 2016; 1(1): 146-151.
- Erhabor, O., Adias, T.C. and Hart, M.L. (2010). Effects of falciparum malaria on the indices of anaemia among pregnant women in the Niger Delta of Nigeria. J of Clin Medicine and Res. 2010; 2(3): 35-41.
- Lee, H.K., Lim, J., Kim, M., Lee, S., Oh, E.J., Lee, J., Kim, J.Y., Han, K., Lee, E.J., Kang, C.S. and Kim, B.K. AnnTrop Med Parasitol. 2001; 95: 31-39.
- Adesina, K.T., Balogun, O.R., Babatunde, A.S., Sanni, M.A., Fadeyi, A. and Aderibigbe,S. Impact of malaria parasitemia on haematological parameters in pregnant women at booking in ilorin, Nigeria. Tren Med Res. 2009; 4: 84-90.
- Angus, B.J., Chotivanich, K., Silamut, K., Ruangveerayuth, R. and Hardeman, M.R. Red blood cell deformability as a predictor of anemia in severe falciparum malaria. Am J of Trop and Med Hyg. 1999; 60: 733-737.