Information
Journal Policies
Surgical Uterine-Sparing Management of Adenomyosis: Techniques and Review of Literature
Andres Vigueras Smith1*, Ramiro Cabrera2, Monica Tessmann Zomer1, Carlos Trippia3, William Kondo1
2.Department of Minimally Invasive Surgery, Angels Hospital, City of Mexico, Mexico.
3.Department Department of Radiology, Nossa Senhora das Graças Hospital, Curitiba, Brazil.
Copyright : © 2018 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objectives: To analyze the current available data on the different conservative surgical techniques for the management of focal and diffuse adenomyosis.
Methods: A comprehensive review of literature was made of publications in English on PubMed and Google Scholar. The studies utilized were then selected by one author in line with the aim of this review.
Results: The uterine-sparing surgical treatment of adenomyosis could be classified in three groups regarding the extension of the remotion of healthy myometrium and the integrity of the uterine wall: Type I: The complete resection of the Adenomyosis (Including Adenomyomectomy and Cystectomy); Type II: Citoreductive surgery (Partial Adenomyomectomy); and Type III: Non-excisional techniques. Many surgical approaches and variants has been described for each technique, predominantly for laparotomy approach. Even when the quality of evidence about benefits of these types of surgery (infertility and symptoms control) remains poor, an 80 % to 85 % of dysmenorrhea and 50 % to 75 % of AUB symptoms improvement is obtain, regardless the technique performed. A important benefit in terms of subfertility - infertility is achieved, with mean pregnancy rates (at 1 year postoperative) of 50 % (Partial remotion) and 65 % (Complete remotion), significantly higher in younger patients. Up to date, no specific technique or approach showed higher benefits in intra or postoperative outcomes. No intra or post operative differences has been found between laparotomy or laparoscopy, therefore laparoscopy is a feasible and effective approach, with all the benefits of minimally invasive surgery.
Abbreviations: MRI: Magnetic resonance imaging; AUC: Area under the curve; TVUS: Transvaginal ultrasound; COC: Combined oral contraceptive; NSAIDs: Non-steroidal anti-inflammatory drugs; HIFU: High frequency ultrasound; RR: Relative risk; IVF: In-vitro fertilization; ICSI: Intra-cytoplasmatic sperm injection; OR: Odds ratio; PR: Pregnancy rate; AUB: Abnormal uterine bleeding; ART: Assisted reproductive techniques.
Adenomyosis, Laparoscopic surgery, Adenomyomectomy, Conservative treatment, Non-Radica,Gynecology, Obstetrics
1. Objective
To analyze the current information related to conservative surgical treatment of adenomyosis, focused in the surgical techniques described for both focal and diffuse adenomyosis, and their effect in symptoms control and fertility improvement.
2. Method
A comprehensive review of the literature was performed with an extensive search of all the English publications on PubMed and Google Scholar related to adenomyosis and the non-radical surgical management. We included all studies found under the search of following keywords: Adenomyosis AND Laparoscopic Surgery OR Myometrectomy OR Adenomyomectomy OR Non-radical Surgery.
One author independently made a selection of relevant abstracts according to the aim of this review. The primary objective was to know and describe the main surgical techniques and analyze the post operative fertility and symptoms-control outcomes.
3. Introduction, Definitions And Epidemiology
Over a century has passed since adenomyosis was described for the first time, and yet it remains a disease difficult to understand and approach for both clinicians and investigators.
Initially named ‘Adenomyoma’ was firstly described in 1860 by the German pathologist Karl Freiherr Von Rokitansky, who found endometrial glands within the myometrium and designated this finding as a ’Cystosarcoma Adenoids Uterinum’[1].
The first modern definition of the disease was described by the American Journal of Obstetrics and Gynecology of 1972: “Adenomyosis may be defined as the benign invasion of endometrium into the myometrium, producing a diffusely enlarged uterus which microscopically exhibits ectopic non-neoplastic, endometrial glands and stroma surrounded by the hypertrophic and hyper-plastic myometrium” [2,3].
Final diagnosis is histopathological, showing the classic ectopic endometrium (Glands and/or Stroma) more than 2.5 mm in-deep in the myometrium or more than one microscopic field at 10X magnification from the endometrium - myometrium junction, surrounded by bundles of hypertrophic smooth muscle cells in a “collar fashion”[4].
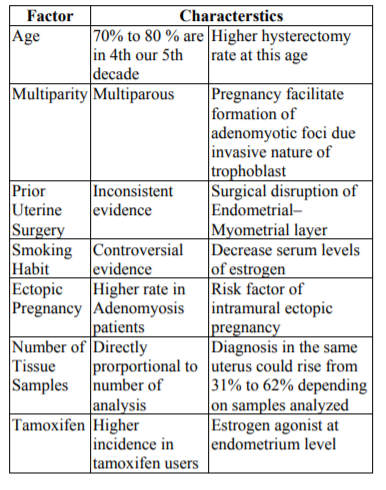
The largest series evaluating the prevalence of the disease found a wide range, between 5% to 70%, with a mean histological diagnosis of 20% to 30%. These wide variations are explained by a combination of patients characteristics and diagnostics method. Nevertheless, clear risk factors have been reported, and are show in Table 1[3].
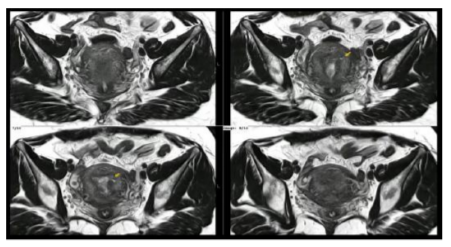
Suspicion is made based on clinical and imaging findings, mostly on ultrasound and MRI explorations.
Clinical manifestations commonly include dysmenorrhea and menorrhagia, but also dyspareunia, chronic pelvic pain and infertility have been reported[5].
The specificity of preoperative diagnosis of adenomyosis based on clinical findings is poor, ranging from 2% to 26% and many cases of adenomyosis go undetected: Therefore, the understanding ofthe prevalence and clinical impact is impaired[6].
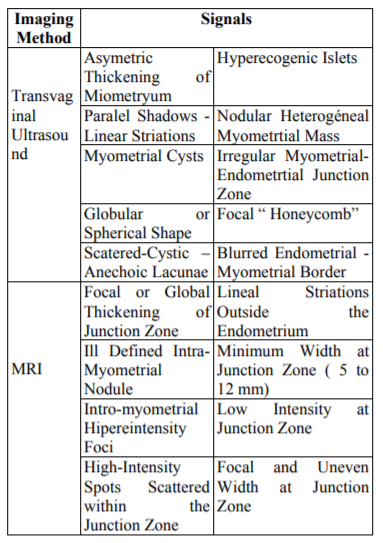
The classic ultrasound findings established by numerous authors over time are shown in Table 2.
The major studies show a sensibility, specificity and AUC of 72%, 81% and 0.85 respectively[7]. A meta-analisis published by Meredith in 2009 confirm the TVUS as a moderately accurate but a easy, wide available and cost-effective preoperative diagnostic test[8].
Meanwhile, MRI show better results because it is less operator dependent, show reliable findings and had better correlation with histology analysis .Common findings are shown in Table 2. The sensibility, specificity and AUC reported are 77%, 89% and 0.92 respectively[7].
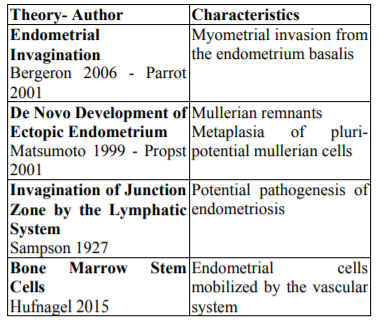
Many theories has born trying to explain the origin of the disease, nevertheless four are the most accepted and are showed in Table 3[7].
The first line treatment for adenomyosis is medical, based on hormone therapy and symptomatic drugs, including NSAIDs, GnRH agonists, Danazol, COC, among others. In when such treatments are contraindicated, failed or when patient seek for fertility, surgical treatment is indicated[9].
Since Hyams first suggested the use and benefits of the conservative surgical management for adenomyosis in 1952, many surgical techniques have been described[10].
The aforementioned passages highlight the current level of conceptual understanding regarding the techniques to treat adenomyosis, however, on a global scale the general level of knowledge surrounding these procedures is still in the development stages for laparoscopy. Therefore the intent of this review analyses the available proposals of surgical uterine-sparing techniques focused on laparoscopic approach for the treatment of symptomatic adenomyosis, and tries to assess the effect of each type of surgical treatment on future fertility and symptoms control. We start giving definitions and epidemiological information. Afterward, reviews of conservative surgical techniques are presented. Finally, we discuss these techniques in terms of fertility and symptoms control issues and draw conclusions.
4. Classification Of Conservative Uterine-Sparing Surgical Techniques
Broadly talking, surgical management of adenomyosis can be separated in two main options: Radical and Conservative. Radical include the hysterectomy, a invasive, definitive and no fertility sparing approach.
Before discussing the conservative surgical techniques, classification can be made based on the extent of removal of adjacent healthy myometrium and the preservation of the uterus integrity[9].
The basic principle of all “uterine-sparing” techniques is that most of the time a piece of healthy myometrium is resected, despite the technique preformed.
Therefore, three types of conservative surgical treatments had been established:
1. Complete Excision of Adenomyosis (Type I). Includes Adenomyomectomy and Cystectomy. Preferably used in cases of localized adenomyosis (Adenomyoma) but also in selected cases of more diffuse adenomyosis with reconstruction of the uterine wall. This includes the complete removal of all recognizable macroscopic disease. The integrity of uterine wall is maintained. It is the most radical approach and offers the major advantages among all[11-14].
2. Partial Adenomyomectomy (Type II). Cytoreductive surgery used in cases of diffuse adenomyosis, including partial removal of clinically recognizable disease including as much of macroscopic lesions as possible. Generally, it is more related to less symptom control efficacy and higher postoperative recurrence rate[12,14,15].
3. Non-Excisional Techniques (Type III). A heterogeneous group of procedures where removal of adenomyotic tissue is not included. This Include laparoscopic, histeroscopic and combined procedures, using devascularization, coagulation and ablation techniques[14,15].
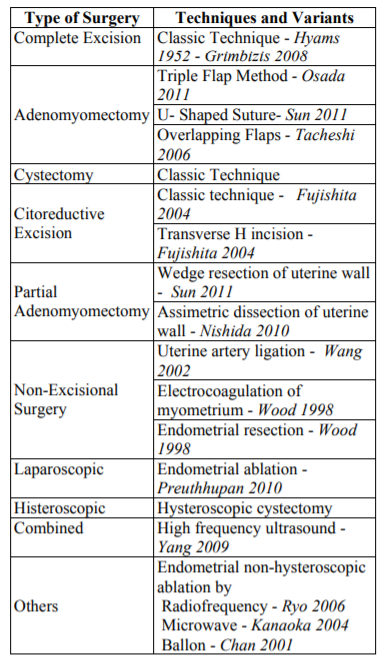
A resume of these surgeries, specific techniques and variants is described in Table 4
Differences exist between these three types of surgery, clearly presented by Horng in 2014[9].
A comparison between type I and II surgeries are presented in Table 5. (Showed Adapted from Horng et al. 2014)
The election of the best surgical approach depends in many variables, but mainly in the anatomical locations of the disease diagnosed in the preoperative imaging exams. (Focal or Diffuse).
The Adenomyomectomy technique is based on a completely new idea that differs from conventional surgical procedures. The method involves reconstruction of the uterine wall surgical defect using normal uterine wall muscle. The technique is not only effective for diffuse, but also for focal adenomyosis, and it has the potential to contribute to the prevention of uterine ruptures during future pregnancies. The classic and the overlapping flaps techniques (with their variants) will be described[16].
The Adenomyomectomy (open or laparoscopic) includes the same steps performed in a myomectomy, including a linear myometrial incision and find (and remain) in the right cleavage plane[11].
The major problem here is that Adenomyoma show intra-operative Ill- defined limits and absence of a pseudo-capsule around him, this difficult the surgical approach since no clear cleavage plane is obtained during dissection. Hence, bloodless and complete resection procedure turns challenging.
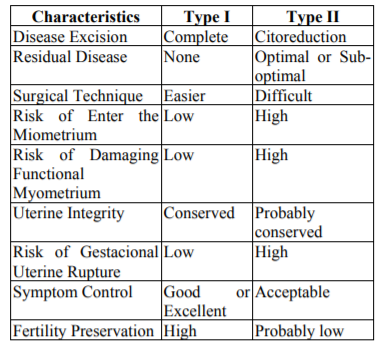
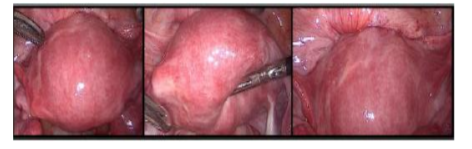
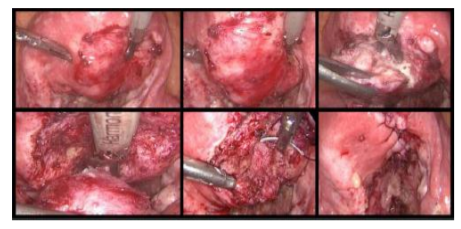
The technique could be performed either by laparoscopy or laparotomy, it includes the following consecutive 6 steps clearly presented by Grimbizis in 2014:1. Recognition of the lesion's location and borders by inspection and direct palpation (or laparoscopic grasper haptic feedback); 2.Longitudinal incision of the uterine wall direct over the lesion; 3. Sharp and blunt dissection of the adenomyoma with scissors, graspers, and/or diathermy similarly to performed during a myomectomy;4.Suturing of the uterine cavity using 4-0 synthetic absorbable suture (if the endometrium cavity was opened); 5.Closure of the uterine wall with 1-0 synthetic absorbable suture layer by layer; 6.Closure of the serosa layer with 4-0 or 5-0 absorbable sutures[9,11]. As we see,the surgical technique is quite similar to myomectomy, and is presented in Figure 1.
Ending the procedure, the key steps are always perform a sero-muscular closure (one or two planes) and use an adequate morcelation technique under protection. The use of intra-operative ultrasonographic guidance could improve the result of the resection.
There are two major modifications for this classic technique: The U-shaped Suturing proposed by Sun in 2011 and the Overlapping Flaps of Takeshi in 2006, both for the laparoscopic approach.
The U-shaped Suturing consists in the approximation of the uterine walls using U-shape sutures at the muscle layer, trying to completely approximate the “walls cave like wound” resulted after disease resection. Posteriorly, the sero-muscular layer is closed by figure eight stitches, using the same threads as the classic technique[11].
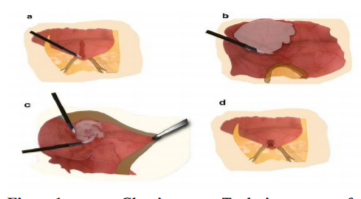
In the Overlapping Flaps, a transverse incision is made over the adenomyotic tissue, and the lesion is excised with a monopolar needle, following the macroscopic edges of the disease, trying to respect all the clinical adenomyosis.
The remaining sero-muscular layer is overlapped and sutured to counteract the lost muscle layer of the uterus resulted after the resection[11]. The surgical technique is showed in Figure 2.
Osada in 2011present the Triple-Flap Method, another Type I procedure described for laparotomy, startingwith a transverse suprapubic incision, leaving after the excision, 1cm margin of tissue above the endometrium and a 1 cm margin of tissue below the serosal surface. This surgical technique is described for laparotomy and it is performed following 6 consecutive steps: 1. Use a rubber tourniquet placed in the Itsmical area for hemostasis; 2. Bisection of the uterus in the midline and in the sagittal plane with a cold scalpel until the uterine cavity is reached and opened; 3.Introduction of the index finger to guide excision of the adenomyotic tissue; 4.Use of a Martin forceps to resect the adenomyotic tissue from healthy surrounding myometrium; 5.Closure of the endometrium with multifilament synthetic reabsorbable thread (3–0 Vicryl); 6. Closure of myometrium walls and uterine serosa: on one side of the bisected uterus they are approximated in the antero-posterior plane with interrupted sutures of 2–0 Vicryl, while the contra lateral side is brought over the reconstructed first side in such a way as to cover the sero-muscular suture line[12].
Special care must be taken for do not overlap the suture lines; only myometrial tissue flaps must overlap. To perform this, the serosal surface of the underlying flaps must first be stripped, that is, the myometrium of the underlying flap must be denuded of serosa[12].
Since the major part of these techniques and variations are described for laparotomy, no detailed description of these procedures is presented here.
Many techniques has been described for citoreductive treatment, and four are classically presented: The Classic Technique, The Transverse H Incision, The Wedge resection of the Uterine Wall and The Asymmetric Dissection of the Uterine Wall[11].
This technique is performed in four consecutive steps starting with a vertical or transverse incision in the middle of uterine wall, recognition and resection of all macroscopic disease and a final wound closure in at least 2 layers, taken care of not leave any uterine defect behind.
This technique consists in a modification of the classic technique, indicated for adenomyosis affecting the anterior uterine wall. Helped by a vasoconstriction agent, a vertical incision is performed followed by two transverse incisions perpendicularly to the initial one, along the upper and lower edges of the uterus. Resection of all macroscopic adenomyotic tissue is performed in a slicing fashion, helped by direct manual palpitation. Closure is finally made in at least two layers of interrupted stitches.
It is another technique, that could be performed in both laparotomy and laparoscopy approaches. Consist in a wedge resection of the sero-muscular layer related to the adenomyotic lesion. Afterward, closure is performed similarly to the classic technique previously described.
It is an approach where the uterine wall is dissected longitudinally using electrosurgery in an asymmetrical fashion, to divide the inside from the outside, preserving both the uterine cavity and uterine arteries. Posteriorly, the myometrium is dissected diagonally until endometrial cavity is opened and adenomyotic tissue is excised using a loop electrode, helped by the index finger inserted into endometrial cavity. Finally, wound closure is performed separately, initially for the endometrial cavity and then for the myometrial and serosal layers.
The following groups of non-excisional techniques have been described in the literature for the uterine-sparing management of adenomyosis. As previously said, removal of adenomyotic tissue is not the aim of the surgery.
These techniques include laparoscopic electro coagulation of the myometrium [15,17,18] and laparoscopic uterine artery ligation[19]. Both are described as fertility sparing techniques, with minor effects in future fertility.
These techniques include operative hysteroscopy[20] , roller ball endometrial ablation [21], trans-cervical resection of the endometrium [22, 23], and endomyometrial resection[15]. Many authors report these techniques as a fertility harming procedures, since all of them will include some degree of damage to endometrium and/or myometrium.
These techniques include ablation of focal adenomyosis with high frequency ultrasound (HIFU)[24], Alcohol instillation under ultrasound guidance (for the treatment of cystic adenomyosis) [25], radiofrequency ablation of focal adenomyosis [26], microwave endometrial ablation [27], and balloon thermo-ablation [28]for diffuse adenomyosis. As well as hysteroscopic techniques, these techniques have been reported as fertility harming.
Also, the combination of excisional (Type II) and non- excisional technique (Type III) could be perform in order to improve postoperative results. The main combination will be the laparoscopic resection of adenomyosis after a previous uterine artery occlusion. This occlusion could be transient or permanent[29].
If some part of the adenomyotic tissue is resected, and as Type II procedures, it is nearly impossible to remove all adenomyotic foci. Additionally, concomitant resection of a large amount of healthy myometrium with destruction of functional myometrium cannot be totally avoided[10,30].
5. Symptom Control Outcomes After Conservative Treatment
The main symptoms related to adenomyosis is the dysmenorrhea, menorrhagia and chronic pelvic pain[3].
The clinical effectiveness of conservative uterine-sparing surgery for adenomyosis is reported in the literature as promising. In the review of Gregoris Grimbizis presented in the Fertility and Sterility of 2014, this surgical approach provide significantly improvement of symptom control, achieving reduction of more than 81% of dysmenorrhea and 50% of menorrhagia. Hence, symptoms improvement in more than two-thirds of patients after type I uterine- sparing surgery, and nearly a half of the patients after type II uterine-sparing procedures is reported. Meanwhile for a Type III, the major studies report a reduction in 54% of dysmenhorrea and 73% of AUB[11].
6. Fertility Outcomes After Conservative Treatment
Broadly talking, adenomyosis appears to have a detrimental impact on reproductive performance in terms of decreased clinical pregnancy rate (PR) and increased abortion rate. Nevertheless, clear and direct relationship between adenomyosis and fertility is still a controversial issue.
Some retrospective studies showed an absence of a direct relationship between adenomyosis and the clinical PR, with no significant differences in PR among women with and without adenomyosis[31].
By contrast, prospective evidence are show significantly lower PR in affected patients. In the study of Vercillini and Cosonni in 2014, comparing pregnancy rates in between 98 positive to 567 negative adenomyosis patients, women's with adenomyosis had a significantly lower clinical PR (24/98, 24.5%) than women without adenomyosis (245/567, 43.2%), with a RR of 0.55[31].
The summary of findings in terms of reproductive outcomes in women with and without adenomyosis, demonstrate that patients with the disease has a significantly decreased clinical PR and increased miscarriage rate, contributing to a important decrease in the final live birth rate. These results hardly suggest that there is a strong association between adenomyosis and fertility[32].
But, even though these studies are more balanced towards the side of adenomyosis as an associated factor in the negative impact on a woman's fertility, we must bear in mind that there is no definitive consensus on when to choose between medical or conservative surgery as a primary treatment.
As well, women with adenomyosis submitted to ART seemed to have a lower implantation rate per embryo transfer, PR and live birth rate, but a higher spontaneous abortion rate. Therefore, a significant decrease in the clinical PR is reported (RR 0.72), and a twofold risk of miscarriage is also observed (RR 2.12). These findings suggest that the adenomyotic uterine environment increases the risk of miscarriage independently of oocyte and embryo quality[31].
K.-H.Tsui suggest an approach initially with a long-protocol GnRH agonist suppression for natural conception in women with normal ovarian function, but an urgent IVF procedure in women's with diminished ovarian reserve. When repeat failure of along GnRH agonist-based IVF/ICSI therapy is observed, conservative uterine -sparing surgery of adenomyosis should be indicated, and urgent following long-protocol IVF/ICSI 3 months later after complete treatment it’s also recommended[32,33].
According to published, the uterine-sparing surgical treatment certainly improve the pregnancy outcome. Pregnancy rates are around 60% in women's with Type I and over 46% in women's with Type II surgery. Two-thirds of the women treated with Type II surgery and more than four-fifths of the women treated with Type I surgery had a successful delivery.
Women's with localized adenomyosis after adenomyomectomy had a pregnancy rate that ranged from 48.2% to 77.5%, with a successful delivery rate between 26.8% to 69.0% [24,34].
Although conservative surgery might not have a similarly promising effect on reproductive performance in women with diffuse-type adenomyosis, the PR reported after sparing surgery in this group range between 30% - 40%, amounting to nearly 25% to 30% of the women who had a successful delivery[11,35,36].
Nevertheless, clinician must never forget that the most critical factor of fertility is age, and has been demonstrated thatPR among women with adenomyosis treated with conservative surgery vary significantly according to this variable. A Japanese study published in 2014 including102 patients showed that the clinical PR among women with adenomyosis treated with conservative laparoscopic surgery were 41.3% in those aged 39 years and 3.7% in those aged 40 years, suggesting a direct adverse impact of age (OR 0.77). Therefore, the authors recommended that conservative surgery for adenomyosis could be a beneficial treatment for women who experienced IVF treatment failures, especially those aged 39 years or less[33].
The major benefits of this surgery in both fertility and pain control outcomes, is in those patients with the association of adenomyosis and endometriosis. Hence, efforts must be increase in this specific group.
Finally, surgeons must remember that this surgery could lead to pelvic and endometrial adhesions, uterine cavity deformations and reduction of uterine capacity, all of them potentially harming the fertility success. Hence, a broad preoperative patient counselling, a meticulous surgical technique and a thorough post-operative follow-up is always recommended.
7. Conclusions
Conservative uterine-sparing surgical management of adenomyosis is a safe and feasible, but technically difficult treatment for those women with future pregnancy desire, achieving both good symptom control and preservation of fertility. Independently of the type of surgery performed, significant benefits are reported, with mean control of Dysmenorrhea about 50%, AUB in 50% to 75% and a 12 months postoperative pregnancy rate between 50% to 65%.
The success rate will vary according the type of procedure performed. For Type I surgeries, 82% of pain reduction and 68% of bleeding reduction is reported, with a mean of 60.5% of PR. Meanwhile for type II disease, 84% to 86% of symptoms control is achieved. Finally, Type III techniques will achieve a 54% of pain reduction, 73% of bleeding reduction and 55 % of PR.
Laparoscopy is a feasible approach, and must be used regularly to treat the disease, with lower risk of conversions and intra - post operative complications. The main advantage of laparotomy is the direct palpation and clear recognition of the lesion (and their limits), difficult to achieve by the reduced haptic feedback of the laparoscopy. Nevertheless, this is completely split with the actual MRI exams, clearly delimiting and defining the disease, giving and excellent preoperative work - up, allowing a safely and effectively approach by laparoscopy.
To date, the lack of good evidence mislead the efficacy of different surgical techniques for the treatment of the adenomyosis. It will be necessary to study the benefits of each technique through large-scale randomized studies with adequate follow-ups.
References
- Benagiano G, Brosens I.History of adenomyosis. Best Practice and Research: Clinical Obstetrics and Gynaecology, 20 (4) pp. 449-463.(2006)
- Frankl O. Adenomyosis uteri. American Journal of Obstetrics & Gynecology, Volume 10, Issue 5, 680 – 684. (1925)
- Taran FA, Stewart EA, Brucker S.Adenomyosis: Epidemiology, Risk Factors, Clinical Phenotype and Surgical and Interventional Alternatives to Hysterectomy. Geburtshilfe Und Frauenheilkunde, 73(9), 924–931.(2013)
- Bergeron C, Amant F, Ferenczy A. Pathology and physiopathology of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 20: 511e21.(2006)
- Kim JK, Shin CS, Ko YB, et al.Laparoscopic assisted adenomyomectomy using double flap method. Obstet Gynecol Sci 57: 128-135.(2014)
- Benagiano G, Brosens I, Habiba M. Adenomyosis: a life-cycle approach. Reprod Biomed Online;30:220e32.(2015)
- Argawal M, Mettler L, Alkatou I. A manual of minimally invasive gynecological surgery. Jaypee Brothers Medical Publishers, First Edition (2015)
- Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of trans-vaginal sonography for the diagnosis of adenomyosis: systematic review and metaanalysis. Am J Obstet Gyneco.201:107. e1ee6.(2009)
- Horng HC, Chen CH, Chen CY, et al.Uterine-sparing surgery for adenomyosis and/or adenomyoma. Taiwan J Obstet Gynecol 53: 3-7.(2014)
- Hyams LL. Adenomyosis; its conservative surgical treatment (hysteroplasty) in young women. N Y State J Med 52: 2778-2784.(1952)
- Grimbizis G, Mikos T, Tarlatzis B. Uterus-sparing operative treatment for adenomyosis. Fertility and Sterility 2014, Volume 101, Issue 2, 472 - 487.e8.(2014)
- Osada H, Silber S, Kakinuma T, et al. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod Biomed Online.22:94–9. (2011)
- Protopapas A, Millingos S, Markaki S, et al. Cystic uterine tumors. Gynecol Obstet Invest. 65:275–80. (2008)
- Takeuchi H, Kitade M, Kikuchi I, et al. Diagnosis, laparoscopic management, and histopathologic findings of juvenile cystic adenomyoma: a review of nine cases. Fertil Steril.94:862–8.(2010)
- Wood C. Surgical and medical treatment of adenomyosis. Hum Reprod Update. 4:323– 36.(1998)
- Osada H. Uterine adenomyosis and adenomyoma: the surgical approachFertility and Sterility® Vol. 109, No. 3, March, 0015-028.(2018)
- Phillips DR, Nathanson HG, Milim SJ, et al. Laparoscopic bipolar coagulation for the conservative treatment of adenomyomata. J Am Assoc Gynecol Laparosc. 4:19–24.(1996)
- Wood C. Adenomyosis: difficult to diagnose, and difficult to treat. Diagn Ther Endosc. 7:89–95.(2001)
- Wang CJ, Yen CF, Lee CL, et al. Laparoscopic uterine artery ligation for treatment of symptomatic adenomyosis. J Am Assoc Gynecol Laparosc. 9:293–6.(2002)
- Fernandez C, Ricci P, Fernandez E. Adenomyosis visualized during hysteroscopy. J Minim Invasive Gynecol. 14:555–6.(2007)
- Preutthipan S, Herabutya Y. Hysteroscopic rollerball endometrial ablation as an alternative treatment for adenomyosis with menorrhagia and/or dysmenorrhea. J Obstet Gynaecol Res.36:1031–6.(2010)
- Kumar A, Kumar A. Myometrial cyst. J Minim Invasive Gynecol. 14:395–6.(2007)
- Maia H Jr, Maltez A, Coelho G, et al. Insertion of mirena after endometrial resection in patients with adenomyosis. J Am Assoc Gynecol Laparosc. 10:512–6.(2003)
- Yang Z, Cao YD, Hu LN, et al. Feasibility of laparoscopic high-intensity focused ultrasound treatment for patients with uterine localized adenomyosis. Fertil Steril. 91:2338–43.(2009)
- Furman B, Appelman Z, Hagay Z, et al. Alcohol sclerotherapy for successful treatment of focal adenomyosis: a case report. Ultrasound Obstet Gynecol. 29:460–2.(2007)
- Ryo E, Takeshita S, Shiba M, et al. Radiofrequency ablation for cystic adenomyosis: a case report. J Reprod Med. 51:427–30.(2006)
- Kanaoka Y, Hirai K, Ishiko O. Successful microwave endometrial ablation in a uterus enlarged by adenomyosis. Osaka City Med J. 50:47–51.(2004)
- Chan CL, Annapoorna V, Roy AC, et al. Balloon endometrial thermoablation an alternative management of adenomyosis with menorrhagia and dysmenorrhoea. Med J Malaysia 2001;56:370–3.(2001)
- Kang L, Gong J, Cheng Z, et al. Clinical application and midterm results of laparoscopic partial resection of symptomatic adenomyosis combined with uterine artery occlusion. J Minim Invasive Gynecol. 16: 169–73.(2009)
- Leyendecker G, Kunz G, Kissler S, et al. Adenomyosis and reproduction. Best Pract Res Clin Obstet Gynaecol. 20:523–46.(2006)
- Vercellini P, Consonni D, Dridi D, et al. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod 29:964e77.(2014)
- Kuan-HaoT,Fa-KungL,Kok-Min S.Conservativesurgicaltreatmentof adenomyosistoimprovefertility: Controversial values, indications, complications, and pregnancy outcomes. Taiwanese Journal of Obstetrics & Gynecology 54:635e640.(2015)
- Kishi Y, Yabuta M, Taniguchi F.Who will benefit from uterus-sparing surgery in adenomyosisassociated subfertility?. Fertil Steril;102:802e7.(2014)
- Chang WH, Wang KC, Lee NR,et al. Reproductive performance of severely symptomatic women uterine adenomyoma who wanted preservation of the uterus and underwent combined surgical-medical treatment. Taiwan J Obstet Gynecol. 52:39e45.(2013)
- Wang PH, Fuh JL, Chao HT, et al. Is the surgical approach beneficial to subfertile women with symptomaticextensive adenomyosis J Obstet Gynaecol Res.35:495e502.(2009)
- Huang BS, Seow KM, Tsui KH, et al. Fertility outcome of infertile women with adenomyosis treated with the combination of a conservative microsurgical technique and GnRH agonist: long-term follow-up in a series of nine patients. Taiwan J Obstet Gynecol. 51:212e6. (2012)