Information
Journal Policies
The Co-Evaluation of Endometrial Edema and Uterus Inflammation after Erythropoietin Effect on Uterine Ischemia Reperfusion Injury
C. Tsompos1, C. Panoulis2, K Toutouzas3, A. Triantafyllou4, CG. Zografos5, K.Tsarea6, M. Karamperi7, A. Papalois8
2.Department of Obstetrics&Gynecology, Aretaieion Hospital, Athens University, Athens, Attiki, Hellas.
3.Department of Surgery, Ippokrateion General Hospital, Athens University, Athens, Attiki, Hellas.
4.Department of Biologic Chemistry, Athens University, Athens, Attiki, Hellas.
5.Department of Surgery, Ippokrateion General Hospital, Athens University, Athens, Attiki, Hellas.
6.Experimental Research Centre ELPEN Pharmaceuticals, S.A. Inc., Co., Pikermi, Attiki, Hellas.
7.Experimental Research Centre ELPEN Pharmaceuticals, S.A. Inc., Co., Pikermi, Attiki, Hellas.
8.Experimental Research Centre ELPEN Pharmaceuticals, S.A. Inc., Co., Pikermi, Attiki, Hellas.
Copyright : © 2018 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Aim: This study co-evaluated the 2 quoted histologic variables after erythropoietin (Epo) administration. The calculation was based on the results of 2 preliminary studies, each one evaluating a respective histologic variable of endometrial edema (EE) or uterus inflammation (UI) in an induced ischemia reperfusion animal experiment.
Materials and Methods: The 2 main experimental endpoints at which the EE and UI scores were evaluated was the 60th reperfusion min (for the groups A and C) and the 120th reperfusion min (for the groups B and D). Specially, the groups A and B were processed without drugs, whereas the groups C and D after Epo administration.
Results: The first preliminary study showed that Epo non significantly enhanced the EE scores by without lesions alterations 0.0272727+0.20195934 (p-value=0.8898). The second preliminary study showed that Epo non significantly restored the UI scores by without lesions alterations 0.0727273+.32239888 (p-value=0.6439). These 2 studies were co-evaluated since they came from the same experimental setting. This study co-evaluated the combined diagnostic values of both variables together.
Conclusions: Epo has a hardly enhancing potency of these histologic parameters without lesions alterations 0.05 [-.2504947 .3504947] (p-values=0.7381) since they were co-evaluated together.
ischemia, erythropoietin, endometrial edema, uterus inflammation, reperfusion,Gynecology, Obstetrics
1. Introduction
Erythropoietin (Epo) was investigated whether having antioxidant capacities. 2 histologic variables in a uterine ischemia reperfusion (UIR) experiment was tested for this purpose. The one variable was that of endometrial edema (EE) but was enhanced by without lesions 0.0272727+0.20195934 (p-value=0.8898)[1]. The other variable was that of uterus inflammation (UI) and was restored by without lesions - 0.0727273+.32239888(p-value=0.6439)[2].
Although Epo is met in over 29,662 published biomedical studies, only a 3.48% of them negotiate its antioxidant capacities. The present experimental work tried to co-evaluate these EE and UI variables together and to compare its outcome with each one separately, from the same rat induced UIR protocol.
2. Materials And Methods
The study did not include live subjects but animals. Thus, it received 2 ethics committee approvals under the 3693/12-11- 2010 & 14/10-1-2012 numbers fully following the tenants of the Declaration of Helsinki. The granting company, the experiment location and the Pathology Department are mentioned in preliminary references[1,2]. The human animal care of Albino female Wistar rats, the 7 days pre-experimental ad libitum diet, the non-stop intra-experimental anesthesiologic techniques, the acidometry, the electrocardiogram and the oxygen supply and post-experimental euthanasia are also described in preliminary references. Rats were 16 – 18 weeks old. They were randomly assigned to four (4) groups consisted in N=10. The stage of 45 min ischemia was common for all 4 groups. Afterwards, reperfusion of 60 min was followed in group A; reperfusion of 120 min in group B; immediate Epo intravenous (IV) administration and reperfusion of 60 min in group C; immediate Epo IV administration and reperfusion of 120 min in group D. The dose height assessment was described at preliminary studies as 10 mg/Kg body mass. Ischemia was caused by laparotomic clamping the inferior aorta over renal arteries with forceps for 45 min. The clamp removal was restoring the inferior aorta patency and reperfusion. After exclusion of the blood flow, the protocol of UIR was applied, as described above for each experimental group. Epo was administered at the time of reperfusion; through inferior vena cava catheter. The EE and UI scores were determined at 60th min of reperfusion (for A and C groups) and at 120th min of reperfusion (for B and D groups). Relation was rised between animals’ mass with neither EE scores (p-value=0.0861) nor with UI ones (p-values=0.7954). The pathologic score grading was maintained the same as in preliminary studies: (0-0.499) without lesions, (0.5-1.499) the mild lesions, (1.5 -2.499) the moderate lesions and (2.5-3) the serious lesions damage.
Injury Control groups
The 20 control rats were the same for preliminaries and this study.
1. Group A
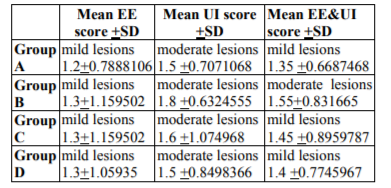
Reperfusion which lasted 60 min concerned 10 controls rats of combined EE and UI (EE&UI) score as the mean of EE score and UI one (Table1).
2. Group B
Reperfusion which lasted 120 min concerned 10 controls rats of combined EE&UI (cEE&UI) score as the mean of EE and UI one (Table1). Erythropoietin group The 20 Epo rats were the same for preliminaries and this study.
3. Group C
Reperfusion which lasted 60 min concerned 10 Epo rats of cEE&UI score as the mean of EE score and UI one (Table1).
4. Group D
Reperfusion which lasted 120 min concerned 10 Epo rats of cEE&UI score as the mean of EE score and UI one (Table1).
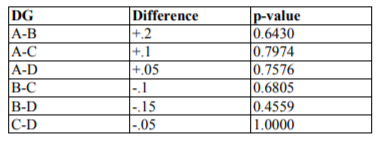
Every cEE&UI groups score was compared with each other from 3 remained groups applying Wilcoxon signed-rank test (Table2). Then, the generalized linear models (glm) were applied with dependant variable the cEE&UI scores, and independent variables the Epo administration or no, the reperfusion time and their interaction.
3. Results
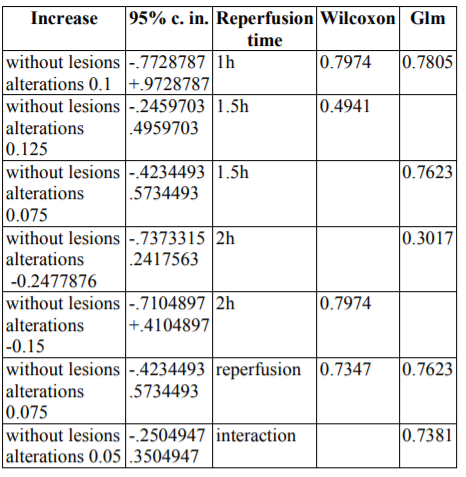
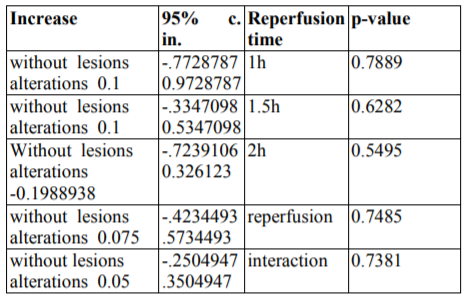
Epo administration non-significantly enhanced the cEE&UI scores by without lesions alterations 0.1 [-.3347098 0.5347098] (p=0.6282) after co-calculation by both Wilcoxon signed-rank test and glm methods. Similarly, reperfusion time hardly enhanced the cEE&UI scores by without lesions alterations 0.075 [-.4234493 .5734493] (p=0.7485) after co-calculation by the same methods.
However, erythropoietin administration and reperfusion time together also hardly enhanced the cEE&UI scores by without lesions alterations 0.05 [-.2504947 .3504947] (p=0.7381). A concise form of the above findings is depicted at table4.
4. Discussion
Thaete LG et al used[3] Pep-1 (inhibits low-molecular-weight hyaluronan (LMW-HA) due to binding to toll-like receptor 4 [TLR4]). TLR4 was shown to have a regulatory role for two anti-inflammatory cytokines: interferon-B1 decreased only in wild-type mice (P< 0.01) and interleukin-10 increased only in TLR4-deficient mice (P< 0.001), in response to UIR. Pep-1 completely prevented the UIR induced fetal growth restriction (FGR) (P< 0.001), indicating a potential role for the endogenous TLR4 ligand LMW-HA in UIR induced FGR. FGR is dependent on TLR4 and endogenous ligand(s), including breakdown products of HA. In addition, TLR4 may play a role in preventing pregnancy loss after UIR. Reiter RJ et al described[4] placenta, in particular, often as a site of excessive free radical generation due to less than optimal adhesion to the uterine wall, which leads to either persistent hypoxia or intermittent hypoxia and reoxygenation, processes that cause massive free radical generation and organ dysfunction. This may contribute to pre-eclampsia and other disorders which often complicate pregnancy. Melatonin has ameliorated free radical damage to the placenta and to the fetus in experiments using non-human mammals. Optimal circadian rhythmicity in the mother is important since her circadian clock, either directly or indirectly via the melatonin rhythm, programs the developing master oscillator of the fetus. Experimental studies have shown that disturbed maternal circadian rhythms, referred to as chronodisruption, and perturbed melatonin cycles have negative consequences for the maturing fetal oscillators, which may lead to psychological and behavioral problems in the newborn. Μelatonin, of both pineal and placental origin, has essential functions in fetal maturation and placenta/uterine homeostasis. Circadian clock genes, which are components of all cells including those in the peripheral reproductive organs, have important roles in reproductive and organismal (fetal and maternal) physiology. Indoleamine may have utility in the treatment of pre-eclampsia, intrauterine growth restriction, placental and fetal IR due to the potent antioxidant actions of melatonin, coupled with its virtual absence of toxicity. The propensity for parturition to occur at night may relate with the synergism between the nocturnal increase in melatonin and oxytocin. Sahin S et al indicated5 that immunosuppressant tacrolimus reduces oxidative damage in rat UIR Histologic evaluation revealed that tacrolimus attenuates the inflammatory response and protects the tissue damage induced by UIR in rats. Alawadhi F et al improved6 fertility after bone marrow derived stem cells (BMDSC) transplant in Asherman's Syndrome mice, demonstrating a functional role for these cells in uterine repair. BMDSC transplantation is a potential novel treatment for Asherman's Syndrome and may also be useful to prevent a murine model of Asherman's syndrome after uterine injury. Trifonova EA et al studied[7] a cluster of 63 differentially expressed genes (DEG) whose expression level is increased in patients with preeclampsia includes not only the known candidate genes that have been identified in many other genome-wide studies (e.g., LEP, BHLHB2, SIGLEC6, RDH13, BCL6), but also new genes (ANKRD37, SYDE1, CYBA, ITGB2, etc.), which can be considered as new biological markers of preeclampsia and are of further interest. The results of a functional annotation of DEG show that the development of preeclampsia may be related with a stress response, immune processes, the regulation of cell-cell interactions, intracellular signaling cascades, etc. Iran-Nejad A et al[8] found uterus weight increased by estradiol (P < 0.05) after renal IR injury in female rats. Drobyshevsky A et al showed[9] a significant 3.72-fold decrease in maternal placental perfusion in reperfusion-reoxygenation phase in the saline than the antioxidant group dynamic contrast enhanced (DCE) MRI, relative to pre-occlusion values correspondingly. 31% systematic underestimation of true perfusion in placenta by steepest slope DCE MRI is significant in a rabbit model of fetal antenatal hypoxia-ischemia. Vafapour M et al found uterus weight decreased[10]significantly in female rats treated with GABA. Atalay YO et al found[11] remifentanil to protect the UIR and can be used safely in uterus transplantation in exposed rats. Talebi N et al[12] found the uterus weight increased significantly after estradiol administration (P < 0.05) in ovariectomized rats. Tang Y et al indicated13 that soy isoflavone (SI), a soy-derived phytoestrogen, which has similar chemical structure to endogenous estrogen-estradiol; protects myocardial IR injury in ovariectomized rats through increasing PI3K/Akt/eNOS signal pathway and decreasing oxidative stress. Ingles J et al defined[14] the preconditioning as "the preparation for a subsequent action." The unfolded protein response (UPR) is a cellular stress response controlled at the level of the endoplasmic reticulum. However, in the context of remote preconditioning, activation of these intracellular molecular pathways must result in the extracellular transmission of adaptive signals to remote targets. The activation of the UPR in the pregnant uterine myocyte may be associated with increased uterine myocyte quiescence and normal gestational length. A gestational stress-induced uterine paracrine secretome - for example, glucose-regulated protein 78, with preconditioning-like properties - acts to promote both local and systemic tolerance to the ensuing gestational insults, allowing for the maintenance of uterine quiescence. In this context, preterm labor may be the result of a pregnant uterus experiencing a stress it cannot accommodate or when it is unable to host an appropriate UPR resulting in insufficient preconditioning and a diminished local and systemic capacity to tolerate pregnancy-dependent increases in normal gestational stress; in order to prolong uterine quiescence in pregnancy. Tricard J et al revealed15 a moderate inflammation of the endometrium and serosa at 90 min following reperfusion in the 3-h group and severe inflammation in the 24-h group. These first macroscopic and histological results suggest that the uterus is an organ with a good tolerance to extended cold ischemic storage before transplantation in ewes. Aslan M et al found[16] the cellular damage of uterus reduced in oxytocin and kisspeptin administered IR group than only kisspeptin injected IR group and IR group. The present results suggest that exogenous application of oxytocin and kisspeptin can have antioxidant effects on the uterus. A numeric evaluation17 of the Epo efficacies was provided by a meta-analysis of 34 seric variables of complete blood count and blood chemistry tests versus reperfusion time coming from the same experimental setting (table5).
5. Conclusion
Epo has a hardly enhancing potency for endometrial edema and uterus inflammation together (p-values=0.7381) discouraging for beneficial usage in situations such as fetal growth restriction, pregnancy loss, pre-eclampsia, intrauterine growth restriction, placental and fetal IR, fertility, Asherman's syndrome, uterus transplantation, preterm labor, endometritis.
Acknowledgement
Acknowledged in preliminary studies.
References
- C Tsompos, C Panoulis, K Toutouzas, G Zografos, A Papalois. The effect of erythropoietin on endometrial edema during ischemia–reperfusion injury in rats. Journal of Histotechnology. 2016; 39(3): 97-101.
- Τsompos C, Panoulis C, Τοutouzas K, Triantafyllou A, Ζografos G, Papalois A. The effect of erythropoietin on uterus inflammation during ischemia reperfusion injury in rats. Čes. Gynek. 2016; 81(5): 342-348.
- Thaete LG, Qu XW, Jilling T, Crawford SE, Fitchev P, Hirsch E, Khan S, Neerhof MG. Impact of toll-like receptor 4 deficiency on the response to uterine ischemia/reperfusion in mice. Reproduction. 2013 Apr 29;145(5):517-26.
- Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Reprod Update. 2014 Mar-Apr;20(2):293-307.
- Sahin S, Ozakpinar OB, Ak K, Eroglu M, Acikel M, Tetik S, Uras F, Cetinel S. The protective effects of tacrolimus on rat uteri exposed to ischemia-reperfusion injury: a biochemical and histopathologic evaluation. Fertil Steril. 2014 Apr; 101(4):1176-82.
- Alawadhi F, Du H, Cakmak H, Taylor HS. Bone Marrow-Derived Stem Cell (BMDSC) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS One. 2014 May 12;9(5):e96662.
- Trifonova EA, Gabidulina TV, Ershov NI, Serebrova VN, Vorozhishcheva AY, Stepanov VA. Analysis of the placental tissue transcriptome of normal and preeclampsia complicated pregnancies. Acta Naturae. 2014 Apr; 6(2):71-83.
- Iran-Nejad A, Nematbakhsh M, Eshraghi-Jazi F, Talebi A. Preventive role of estradiol on kidney injury induced by renal ischemia-reperfusion in male and female rats. Int J Prev Med. 2015 Mar 20; 6:22.
- Drobyshevsky A, Prasad PV. Placental perfusion in uterine ischemia model as evaluated by dynamic contrast enhanced MRI. J Magn Reson Imaging. 2015 Sep; 42(3):666-72
- Vafapour M, Nematbakhsh M, Monajemi R, Mazaheri S, Talebi A, Talebi N, Shirdavani S. Effect of Γ-aminobutyric acid on kidney injury induced by renal ischemia-reperfusion in male and female rats: Gender-related difference. Adv Biomed Res. 2015 Jul 27; 4:158.
- Atalay YO, Aktas S, Sahin S, Kucukodaci Z, Ozakpinar OB. Remifentanil protects uterus against ischemia-reperfusion injury in rats. Acta Cir Bras. 2015 Nov; 30(11):756-61.
- Talebi N, Nematbakhsh M, Monajemi R, Mazaheri S, Talebi A, Vafapour M. The Protective Effect of γ-aminobutyric Acid on Kidney Injury Induced by Renal Ischemia-reperfusion in Ovariectomized Estradiol-treated Rats. Int J Prev Med. 2016 Jan 11; 7:6.
- Tang Y, Li S, Zhang P, Zhu J, Meng G, Xie L, Yu Y, Ji Y, Han Y. Soy Isoflavone Protects Myocardial Ischemia/Reperfusion Injury through Increasing Endothelial Nitric Oxide Synthase and Decreasing Oxidative Stress in Ovariectomized Rats. Oxid Med Cell Longev. 2016; 2016:5057405.
- Ingles J, Kyathanahalli CN, Jeyasuria P, Condon JC. Thinking Outside the Box. J Cardiovasc Pharmacol Ther. 2017 Jul;22(4):337-346.
- Tricard J, Ponsonnard S, Tholance Y, Mesturoux L, Lachatre D, Couquet C, Terro F, Yardin C, Marquet P, Piccardo A, Gauthier T. Uterus tolerance to extended cold ischemic storage after auto-transplantation in ewes. Eur J Obstet Gynecol Reprod Biol. 2017 Jul; 214:162-167.
- Aslan M, Erkanli Senturk G, Akkaya H, Sahin S, Yılmaz B. The effect of oxytocin and Kisspeptin-10 in ovary and uterus of ischemia-reperfusion injured rats. Taiwan J Obstet Gynecol. 2017 Aug;56(4):456-462.
- Τsompos C, Panoulis C, Τοutouzas K, Triantafyllou A, Ζografos G, Papalois A. The Acute Effect of Erythropoietin on Mean Platelet Volume Levels during Hypoxia Reoxygenation Injury in Rats. Med Bull Haseki 2016; 54:199-206.