Information
Journal Policies
Papillary Thyroid Carcinoma Arising within a Mature Cystic Teratoma of the Ovary: A Report of Two Cases with Long-Term Follow Up
Marko Klaric1, Emina Babarovic2*, Senija Eminovic2, Ani Mihaljevic Ferrari3, Alemka Brncic-Fischer1, Herman Haller1
2.Department of Pathology, School of Medicine, Rijeka University Hospital Centre, School of Medicine, University of Rijeka, Brace Branchetta 20, Rijeka, Croatia.
3.Clinical Department of Radiotherapy and Oncology, Rijeka University Hospital Centre, School of Medicine, University of Rijeka, Kresimirova 42, Rijeka, Croatia.
Copyright : © 2018 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objectives: To report two patients with papillary thyroid carcinoma arising within mature cystic teratoma with long term follow-up.
Methods: The cases are compared with previous reports of similar entities, with special reference to the treatment modalities and management options in follow-up of these patients.
Results: Final diagnosis of both tumors was established after the initial surgery. Both tumors were histologically classified as the malignant transformation of the thyroid tissue within the mature cystic teratoma, both were papillary type and confined to one ovary. We presented two similar cases, but our therapeutic approach was surgically different. Both patients were treated with surgery alone and are alive with no evidence of the disease after 10 and 5 years, respectively.
Conclusion: Preoperative and intraoperative frozen section diagnosis of malignant transformation within teratoma is very difficult, so optimal management of the patients has not yet been established. Treatment of this tumor should be individualized, but a contour of treatment modalities and management options are visible and our cases may contribute in this achievement.
Malignant transformation, mature cystic teratoma, papillary thyroid carcinoma, ovary, treatment,Gynecology, Obstetrics
1. Introduction
Mature cystic teratoma account for approximately 20% of all ovarian tumors and these tumors contain thyroid tissue in ~15%. Struma ovarii is a monodermal variant of ovarian teratoma that is composed of more than 50% of thyroid tissue and it account for 2.5%- 5% of ovarian teratoma [1]. Malignant struma ovarii was first described by Wetteland in 1956. [2] and since then malignant transformation in struma ovarii has caused great interest due to its numerous unique features [1,3-5]. In the literature, there is no consensus in terminology; the term malignant struma has been used in two ways: struma ovarii with malignant transformation and development of thyroid carcinoma within it and struma ovary with metastatic tissue [1]. Regarding the occurrence of malignancy in struma ovarii, there are few other problems like many cases in literature that have not been correctly classified and the lack of large well documented series [1,5]. In addition, several cases reported as malignant struma ovarii in the older literature were actually examples of ovarian strumal or insular carcinoid [6]. Therefore, it is difficult to say the accurate frequency of malignant struma. By reviewing the literature, papillary carcinoma is the most common thyroid type carcinoma to occur in struma ovarii, follow by follicular carcinoma and recently described new entity highly differentiated follicular carcinoma of ovarian origin, while other types of thyroid carcinoma occurs extremely rare [1,6-12]. Patients with papillary carcinoma ranged from 21 to 68 years old with common symptoms and preoperative findings of pelvic mass and abdominal pain in 40%, menstrual irregularities in 9%, hyperthyroidism in 5-8% and deep vein thrombosis in 4% of patients [10-15].
Considering the rarity of the disease there is no consensus for optimal management of the patients. This report presents two patients with a malignant transformation of the thyroid tissue within the mature cystic teratoma of the ovary with long-term follow up. Both tumors were histologically classified as papillary thyroid carcinoma arising in mature cystic teratoma. These two patients were admitted to our clinic within a short period, but regardless to that were treated in a different way. Therefore, this was a good argument for reviewing the literature because there is still no consensus in terminology, diagnostic criteria, treatment and follow up of these patients.
2. Case Reports
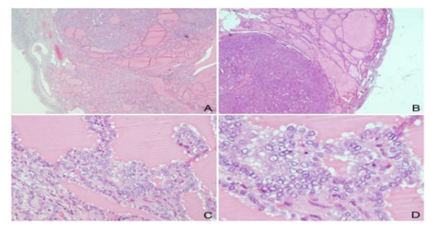
A 57-year old patient, gravida 0, para 0, was admitted into the clinic due to an abdominal ultrasound examination which revealed a tumor of the left ovary. Transvaginal ultrasound examination revealed irregular, predominantly cystic tumor mass of the left ovary measuring 66 x 63 mm in diameter with a smaller solid hyperehogenic part. The uterus was enlarged due to several myomas while the right ovary had a normal appearance. No free fluid in the peritoneal cavity was found. The patient had been previously well, without symptoms and apart from receiving treatment for osteoporosis her medical history was unremarkable. Laboratory findings at the time of admittance were normal. A Serum CA-125 level was 52.6 U/l. Cervical cytologic Pap smear was normal. After the preoperative preparation our patient underwent the operative procedure during which an explorative laparotomy was done. Intraoperatively, uterus was enlarged due to several myomas; the right adnexa were macroscopically normal appearance, while the left ovary was replaced by mobile, predominantly cystic tumor tissue measuring 7 cm in greatest dimension, without adhesions to the surrounding structures. There was no free fluid in the peritoneal cavity. The peritoneal washing was negative. A left adnexectomy was performed and the surgical specimen was sent for an intraoperative histopathologic analysis which revealed cystic mature teratoma filled with suet, hair and a formed tooth. Thereafter, operating procedure was continued and hysterectomy and a right adnexectomy were done. A final histological examination revealed that ovarian tumor tissue was a cystic mature teratoma with a component of thyroid tissue. Within thyroid tissue a partly incapsulated nodule measuring 1.3 cm in greatest dimension was found. Microscopically, the nodule was consisted of solid areas and small thyroid follicles with rare papillary formations lined by one or several layers of neoplastic cells with nuclear features characteristic of papillary thyroid carcinoma (Figure1).
Capsular invasion was present, but there was no lymphovascular invasion and no necrosis in tumor tissue. Definitive diagnosis was papillary thyroid carcinoma arising in mature cystic teratoma. The postoperative course was uneventful. A month after the operation the patient underwent ultrasonographic analysis of the thyroid gland, the analysis of the hormones of the thyroid gland and a whole-body scintiscanning. The reports of the above mentioned tests were all normal and the patient showed no clinical signs of hyperthyroidism. She was further advised to have regular follow-up examinations every 3 months during the first year after surgery, and then every 6 months at the Gynecologic Oncology Outpatient Department (OPD]. Ten years after the surgery, the patient is alive with no evidence of the disease.
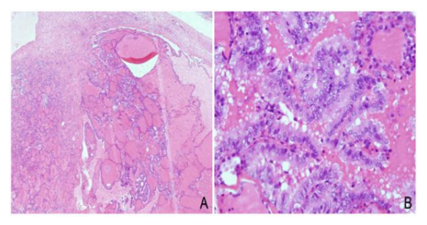
A 35-year old asymptomatic patient, gravida 4, para 1, with a cystic tumor of the left ovary was admitted into the clinic. Her medical history included a partial resection of the right ovary due to a mature teratoma 6 years before hospital admission, and laparoscopic bilateral cystectomy due to follicular cysts of both ovaries, one year after that. Transvaginal ultrasound examination revealed cystic tumor mass of the left ovary measuring 78 x 36 mm, fulfilled with hipoehogenic content, while uterus and the right adnexa had a normal appearance. No free fluid in the peritoneal cavity was found. All laboratory findings, including CA 125, of the patient were normal. Cervical cytologic Pap smear was normal. After the preoperative preparation the patient underwent the operative laparoscopic procedure. Intraoperatively, left ovary was replaced by cystic tumor tissue measuring 8 cm in greatest dimension, with a smooth surface and without adhesions to the surrounding structures. There was no free fluid in the peritoneal cavity and peritoneal washing was negative. The uterus, right ovary, organs and peritoneal surfaces of the abdominal cavity were all macroscopically unremarkable. Since there were no preoperative or intraoperative signs of malignancy, only a left adnexectomy was done and the specimen was not sent for a frozen section analysis. The first postoperative day the patient was discharged from hospital. Pathological gross examination revealed that the left ovary was replaced by predominantly cystic tumor tissue, measuring 8 cm in greatest dimension, with smooth inner walls and filled with suet and hair. A histological examination revealed that ovarian tumor tissue was a cystic mature teratoma with a component of thyroid tissue. Within thyroid tissue, a microscopic focus of papillary thyroid carcinoma measuring 2 mm in greatest dimension was also found (Figure2).
Once again, the definitive diagnosis was papillary thyroid carcinoma arising in mature cystic teratoma. The patient had no complications during the postoperative course, but due to malignant diagnosis she was admitted into our clinic and complete staging was performed one month after the initial operation. The staging procedure included total abdominal hysterectomy with right adnexectomy, infracolic omentectomy with multiple peritoneal biopsies, pelvic and paraaortic lymphadenectomy. All specimens including the peritoneal washing were negative and the disease was staged as FIGO IA, T1aN0M0. The postoperative course was uneventful. The ultrasonographic analyses of the thyroid gland along with thyroid hormones analysis were all normal. Moreover, our patient showed no clinical signs of hyperthyroidism. She was further advised to have regular follow-up examinations every 3 months during the first year after surgery, and then every 6 months at the Gynecologic OPD. Five years after the surgery, the patient is alive with no evidence of the disease.
3. Discussion
Thyroid carcinoma arising within an ovarian mature teratoma is a rare tumor and there are no standard criteria for diagnosis and treatment of such tumor. Most of these tumors are diagnosed postoperatively, after final histopathologic analysis. The histopathologic criteria for diagnosis of malignant transformation in struma ovarii have varied over time, and uniformly accepted criteria for diagnosis of a thyroid-type carcinoma arising in ovarian teratoma have yet to be established. However, criteria for papillary type carcinoma are made on the basis of the same criteria as for the cervical thyroid carcinoma. Criteria for the conventional papillary carcinoma includes true papillary architecture, lined by one or several layers of neoplastic cells with crowded, enlarged, round or oval nuclei, overlapping with ground glass appearance and nuclear grooves [12]. However, it should be stressed that some authors have described ovarian thyroid tumors with subtle atypical nuclear features that are not entirely diagnostic for the follicular variant of papillary thyroid carcinoma, but still have the ability to metastasize [17].
In order to eliminate metastasis from the thyroid gland with secondary involvement of the ovary it is necessary to do further clinical evaluation and an ultrasonographic exam of the thyroid gland [1,3,12].
Serum CA 125 is not a specific marker for diagnosis and observation of the thyroid carcinoma, although it can be elevated in germ cell tumors (teratomas). Nevertheless, there are proofs that tireoglobulin is a good indicator in such tumors. There is a case in which the authors have proved elevated values of tireoglobulin in the blood of the ovarian vein of the ovary affected with the tumor, compared to the blood from another peripheral vein [13]. It can therefore be concluded that tireoglobulin is certainly a good marker for diagnosis and especially for discovery of the recurrence of the disease. So this marker can be very useful in management and follow-up of these patients. The treatment of thyroid carcinoma arising in mature cystic teratoma is still controversial. The surgical treatment ranges from a total abdominal hysterectomy with bilateral salpingoophorectomy, pelvic and paraaortic lymphadenectomy and omentectomy to a conservative surgical procedure that includes unilateral adnexectomy and the preservation of fertility. Adjuvant therapy includes radiotherapy, chemotherapy and the suppression of the thyroid gland. We found different approaches in the literature. Postmenopausal and premenopausal patients that do not want to have children any more should undergo a hysterectomy, bilateral salpingoophorectomy and omentectomy with peritoneal washing as well as pelvic and paraaortic lymphadenectomy, that is, a complete staging [15]. In the case of patients who wish to preserve fertility surgical therapy can include unilateral adnexectomy, but only in those cases where tumor is confined to ovary, has not infiltrated ovarian capsule and there are no proofs of metastasis [18-20].
In case of residual tumor mass after surgical treatment some authors advise a total thyreodectomy and radioablation with I131 as well as in cases with gross extra-ovarian extension of the disease [3,16,21,22] A recent study that examined 57 cases of malignant struma ovarii proved that there was no recurrences in cases treated with adnexectomy, thyroidectomy and I131radioablation and therefore they suggest a multimodal approach to the treatment of this tumors [20]. In cases of recurrence and metastatic disease a chosen therapy would be chemotherapy and radiotherapy combined with the suppression of the thyroid gland [12,15]. The therapy may include a recombinant human TSH in order to achieve higher concentrations of I131 in the tumor [23].
However, the problem lies in the fact that in the majority of cases these tumors are diagnosed postoperatively when the surgery of mature teratoma had already been performed. In such a case, it is impossible to perform a complete staging without the patient undergoing new surgery. Laparoscopic lymphadenectomy can be of use in these cases because of staging [24].
The follow up of patients includes the regular control of the tireoglobulin in the serum, and in the case of elevated values, a scintiscanning with 131I or a PET/CT [25] should be done. A recommended follow-up is a period of at least 10 years. The average time of recurrence is 4 years and there is evidence in literature that some recurrences occurred even 16 years after treatment [26].
Our cases were also diagnosed after surgery when a complete histopathologic diagnosis arrived. In our first case the result of the frozen section, analysis did not reveal the malignancy within the teratoma. During surgery, the first patient underwent a total abdominal hysterectomy with bilateral salpingoophorectomy. The carcinoma was partly incapsulated, confined to one ovary, and the scintiscanning after surgery showed no residual tumor mass or metastasis. The ultrasonographic examination excluded primary thyroid gland carcinoma, and the findings of the hormone of the thyroid gland were normal. Taking all that into account we decided not to perform any radical surgical procedure or adjuvant therapy. The patient was advised to have her tireoglobulin and hormones of the thyroid gland regularly checked. In contrast, the second patient during the first surgical procedure underwent only adnexectomy and although the carcinoma was microscopic in size, a complete staging was indicated. After the complete staging, it was confirmed that the disease is restricted to one ovary and no adjuvant therapy was recommended. The patient was advised to have regular checkups.
4. Conclusion
The above presented cases showed that diagnostic criteria, as well as treatment of the thyroid carcinoma arising within ovarian teratoma are still not defined. Probably due to the rarity of the disease, there is still no accepted procedure for optimal management of the patients. An additional problem is that in most cases the final diagnosis is established after surgery because diagnosis on frozen sections is often not possible, and these tumors do not have a typical symptomatology or reliable diagnostic biomarker. In cases where a metastatic disease in the peritoneal cavity is established, a total staging as well as adjuvant therapy are mandatory. In cases where the tumor is restricted to one ovary, incapsulated and without clear evidence of metastasis in the peritoneal cavity or distant sites, conservative surgical procedure and regular postoperative follow-up are possible. However, in most of these cases it remains unclear whether to repeat the surgical procedure for complete staging of the disease or not. This is the main surgical dilemma. We presented two similar cases that were surgically treated different, but both with good outcome after 10 and 5 years of follow-up, respectively. Treatment of this tumor should be individualized, but a contour of treatment modalities and management options are visible and our cases may contribute in this achievement.
References
- Roth LM, Talerman A. The enigma of struma ovarii. Pathology 2007; 39(1):139–146.
- Wetteland P. Malignant struma ovarii (in Norwegian). Nord Med 1956; 56(43):1568– 1570.
- Willemse PH, Oosterhuis JW, Aalders JG, Piers DA, Sleijfer DT, Vermey A, et al. Malignant struma ovarii treated by ovariectomy, thyroidectomy, and 131I administration Cancer. 1987; 15; 60(2):178-82.
- Berghella V, Ngadiman S, Rosenberg H, Hoda S, Zuna RE. Malignant struma ovarii. A case report and review of the literature. Gynecol Obstet Invest 1997; 43(1):68-72.
- Rosenblum NG, LiVolsi VA, Edmonds PR, Mikuta JJ. Malignant struma ovarii. Gynecol Oncol 1989; 32(2):224-7.
- Roth LM, Talerman A. Recent advances in the pathology and classification of ovarian germ cell tumors. Int J Gynecol Pathol 2006; 25(4):305– 320.
- Roth LM, Karseladze AI. Highly differentiated follicular carcinoma arising from struma ovarii: a report of 3 cases, a review of the literature, and a reassessment of so-called peritoneal strumosis. Int J Gynecol Pathol 2008; 27(2): 213–222.
- Makni SK, Bahri I, Ellouze S, et al. Malignant struma ovarii: a case report. J Gynecol Obstet Biol Reprod [Paris] 2005; 34(8):815–818.
- Shamanna RK, Lee MW, Gaba AR. Struma ovarii with a focus of medullary thyroid carcinoma [poster 24]. Arch Pathol Lab Med 2008; 132(9):1510.
- Fukunaga M, Ishibashi T, Koyama T, Onoue K, Kitai S, Tanaka K, Isonishi S. Malignant Struma Ovarii With a Predominant Component of Anaplastic Carcinoma. Int J Gynecol Pathol 2016; 35(4):357-61.
- Ciccarelli A, Valdes-Socin H, Parma J, Khoo SK, Schoumans J, Colao A, et al. Thyrotoxic adenoma followed by atypical hyperthyroidism due to struma ovarii: clinical and genetic studies. Eur J Endocrinol 2004; 150(4):431-7.
- Devaney K, Snyder R, Norris HJ, Tavassoli FA. Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 cases. Int J Gynecol Pathol 1993; 12(4):333-43.
- Kostoglou-Athanassiou I, Lekka-Katsouli I, Gogou L, Papagrigoriou L, Chatonides I, Kaldrymides P. Malignant struma ovarii: report of a case and review of the literature. Horm Res 2002; 58(1):34-8.
- Matsuda K, Maehama T, Kanazawa K. Malignant struma ovarii with thyrotoxicosis. Gynecol Oncol 2001; 82(3):575-7.
- Makani S, Kim W, Gaba AR. Struma Ovarii with a focus of papillary thyroid cancer: a case report and review of the literature. Gynecol Oncol 2004; 94:835-9.
- DeSimone CP, Lele SM, Modesitt SC. Malignant struma ovarii: a case report and analysis of cases reported in the literature with focus on survival and I131 therapy. Gynecol Oncol 2003; 89(3):543-8.
- Garg K, Soslow RA, Rivera M etal. Histologically bland ‘‘extremely well differentiated’’ thyroid carcinomas arising in struma ovarii recur and metastasize. Int J Gynecol Pathol 2009; 28:222.
- Doganay M, Gungor T, Cavkaytar S, Sirvan L, Mollamahmutoglu L. Malignant struma ovarii with a focus of papillary thyroid cancer: a case report. Arch Gynecol Obstet 2008; 277(4):371-3.
- Hatami M, Breining D, Owers RL, Del Priore G, Goldberg GL. Malignant struma ovarii--a case report and review of the literature. Gynecol Obstet Invest 2008; 65(2):104-7.
- Marti JL, Clark VE, Harper H, Chhieng DC, Sosa JA, Roman SA. Optimal surgical management of well-differentiated thyroid cancer arising in struma ovarii: a series of 4 patients and a review of 53 reported cases. Thyroid 2012; 22(4):400-6.
- Zekri JM, Manifold IH, Wadsley JC. Metastatic struma ovarii: late presentation, unusual features and multiple radioactive iodine treatments. Clin Oncol (R Coll Radiol). 2006; 18(10):768–772.
- Jean S, Tanyi JL, Montone K, McGrath C, Lage-Alvarez MM, Chu CS. Papillary thyroid cancer arising in struma ovarii. J Obstet Gynaecol 2012; 32(3):222-6.
- Rotman-Pikielny P, Reynolds JC, Barker WC, Yen PM, Skarulis MC, Sarlis NJ. Recombinant human thyrotropin for the diagnosis and treatment of a highly functional metastatic struma ovarii. J Clin Endocrinol Metab. 2000; 85(1]:237-44.
- Volpi E, Ferrero A, Nasi PG, Sismondi P. Malignant struma ovarii: a case report of laparoscopic management. Gynecol Oncol. 2003; 90(1]:191-4.
- Nahas Z, Goldenberg D, Fakhry C, Ewertz M, Zeiger M, Ladenson PW, et al. The role of positron emission tomography/computed tomography in the management of recurrent papillary thyroid carcinoma. Laryngoscope. 2005; 115(2]:237-43.
- Hinshaw HD, Smith AL, Desouki MM, Olawaiye AB. Malignant transformation of a mature cystic ovarian teratoma into thyroid carcinoma, mucinous adenocarcinoma, and strumal carcinoid: a case report and literature review. Case Rep Obstet Gynecol. 2012; 2012:269489.