Information
Journal Policies
Efficacy and Safety of Simeprevir-Sofosbuvir Combined Therapy for Treatment oxzf Chronic Hepatitis C Virus Infection
Ashraf M. Osman1, Nahed A. Makhlouf1*, Ahmad F. Alsayed1, Doaa E. Abdel-raouf2
2. Sohag Cardiac and Digestive center - Ministry of Health.
Copyright : © 2018. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Background: Hepatitis C virus (HCV) remains a major health problem worldwide with over 170 million persons chronically infected and a burden of 300000 deaths. HCV Genotype 4 is the predominant Genotype in Egypt. During the past decade, a dual combination of pegylated interferon (PegIFN) and ribavirin (RBV) has represented the standard of care. In the era of directly acting antiviral drugs (DAAs), optimal treatment of HCV Genotype 4 remains, more than ever before, to be defined. Simeprevir (SMV) is a second generation NS3/4A protease inhibitor (PI), active against Genotypes 1, 2, 4, 5 and 6.
Objectives: To determine the efficacy of Simeprevir-Sofosbuvir for treatment of chronic HCV infection among patients attending Sohag Cardiac and Digestive center (Ministry of Health), Upper Egypt.
Patients and Methods: A prospective, hospital based, descriptive study, included 100 patients with chronic HCV infection eligible to receive Simeprevir-Sofosbuvir combination according to the Guidelines of the Ministry of Health.
Results: Early virological response (EVR) was achieved in 99%. At the end of treatment, 94% were negative for HCV (ETVR). Finally, 89% of cases were still negative (sustained virological response) (virological response 12 weeks after end of treatment). The total occurrence of side effects among our study population was 13%, and all of them were mild.
Conclusion: The combination of SOF and SMV is efficacious and well tolerated and represents a good therapeutic option in patients with chronic HCV in Upper Egypt.
Chronic HCV, Simeprevir-Sofosbuvir.
1. Introduction
Worldwide, about 180 million persons were chronically infected with Hepatitis C virus (HCV) [1]. HCV Genotype 4 is the predominant Genotype in Egypt [2]. During the past decade, combination of pegylated interferon (PegIFN) and ribavirin (RBV) was the standard of care. In directly acting antiviral drugs (DAAs) era, optimal treatment of HCV Genotype 4 remains to be defined. Simeprevir (SMV) is a second generation NS3/4A protease inhibitor (PI), active against genotypes 1, 2, 4, 5 and 6. Once- daily tablet orally has demonstrated a favorable safety profile and few drug-drug interactions. Sofosbuvir (SOF) is a nucleotide inhibitor of NS5B had activity on all Genotypes with high barrier to resistance, and it has been proven safe and well-tolerated [3]. Both drugs received approval by the United States Food and Drug for 12 weeks in HCV Genotype 1 infection results in sustained virologic response (SVR) of >90%, regardless of previous treatment or presence of cirrhosis [4]. SMV may cause constipation, hyper- bilirubinemia, rash, pruritus and photo-sensitivity reaction that is the most common side effect [5]. We lack studies of the combined therapy of SMV-SOF for treatment of chronic hepatitis C in Upper Egypt.
2. AIM OF THE WORK
1. To determine the efficacy of SMV-SOF for treatment of chronic HCV infection among patients attending Sohag Cardiac and Digestive center (Ministry of Health).
2. To explore the safety profile of SMV-SOF during treatment of chronic HCV infection.
3. To compare between chronic hepatitis C and cirrhotic regarding treatment efficacy and Administration (FDA). SMV- SOF combination safety profile.
3. PATIENTS AND METHODS
Design: A prospective, hospital based, descriptive study.
This study included 66 patients with chronic HCV infection and 34 patients with HCV related liver cirrhosis (LC) (cirrhotic echo pattern by Ultrasound, and/or varices by upper endoscopy) eligible to receive SMV-SOF combination according to the Guidelines of the Ministry of Health from March 2016 to March 2017.
All patients were subjected to:
1. Clinical Evaluation.
2. Laboratory evaluation including testing for HCV ribonucleic acid (RNA) by quantitative PCR before starting treatment, at the end of 1st month during the course of treatment, at end of treatment, and after end. of treatment by 12 weeks. Sustained virologic response will depend on negative test result after end of treatment by 12 weeks.
3. Laboratory evaluation included liver chemistry, complete blood count every week during 1st two weeks of treatment then every 4 weeks till the end of the course of treatment.
4. Abdominal ultrasonography before start of treatment.
1. Age: 18-75 years.
2. HCV RNA positivity.
3. Any BMI.
4. Treatment naïve or treatment experienced.
1. Direct serum bilirubin >2mg/dl.
2. Serum albumin <2.8g/dl.
3. INR ≥1.7.
4. Platelet count <50000/mm3.
5. Ascites or history of ascites.
6. Hepatic encephalopathy or history of hepatic encephalopathy.
7. HCC, except 4 weeks after intervention aiming at cure with no evidence of activity by dynamic imaging (CT or MRI).
8. Serum creatinine >2.5 mg/dl. If creatinine is between 1.5-2.5 mg/dl, eGFR should be calculated and should exceed 30 ml/min with favorable nephrological consultation.
9. Extrahepatic malignancy except after two years of disease free interval.
10. Pregnancy or inability to use effective contraception.
Sofosbuvir 400 mg/day + Simiprevir 150 mg/day for 12 weeks.
4. ETHICAL CONSIDERATIONS
The study was approved by the Faculty's Ethics Committee. Before enrollment in the study, all participants signed a consent certificate. Before signing, they were able to discuss with the investigator the certificate subjects and the study aim in details. Participants had been clearly informed that refusing to participate in the study would not affect having full benefit of available medical service and treatment. Data were collected by personal interview with participants taking in consideration data confidentiality.
5. STATISTICAL ANALYSIS
Statistical package for social sciences (IBM-SPSS), version 24 IBM- Chicago, USA (May 2016) was used for statistical data analysis. Data were expressed as mean, standard deviation (SD), number and percentage. Mean and standard deviation were used as descriptive value for quantitative data, while number and percentage were used to describe qualitative data. Student t test was used to compare the means between two groups. Pearson Chi square was used to compare percentages of qualitative data. For all these tests, the level of significance (P-value) can be explained as:
- No significance P > 0.05
- Significance P < 0.05
- High significance P < 0.001.
6.RESULTS
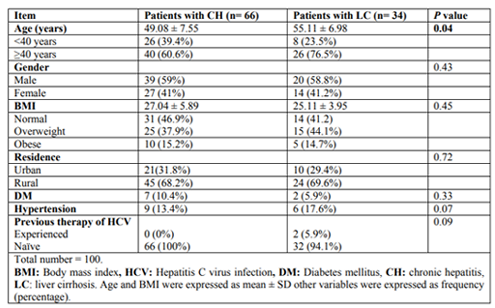
The current study included 66 patients with chronic hepatitis C (CH group) and 34 patients with liver cirrhosis (LC group). The mean age of the study group was 49.08±7.55 years for chronic HCV group (CH group) compared to 55.11±6.98 years among LC group. Approximately two thirds of our study groups were males. The mean BMI of the study group was 27.04±5.89 for CH group and 25.11±3.95 for LC one. Around 40% of our study populations were overweight, and frank obesity ratio was 15%. 98% of the study populations were naïve for HCV antiviral therapy (Table 1).
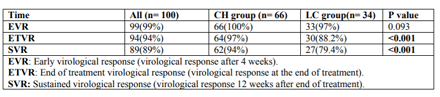
Early virological response (EVR) was achieved in 99% of our cases, with only one case did not show EVR. The end of treatment virological response (ETVR) was seen in 94% of our cases, and sustained virological response (SVR) was maintained in 89% of them (virological Table2. Virological response of the study Groups response 12 weeks after end of treatment). Patients with chronic hepatitis showed significantly higher ETVR and SVR in comparison to those with LC (P value < 0.001) (Table 2).
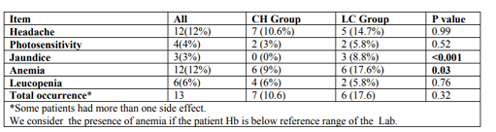
The total occurrence of side effects among our study population was 13%, and all of them were mild. Headache and anemia were the commonest (12% for each), followed by Table3. Side effects of the treatment leucopenia (6%), photosensitivity (4%) and jaundice (3%). Jaundice and anemia were significantly more frequent among LC group compared to CH one (Table 3).
7. DISCUSSION
We lack studies of the combined therapy of SMV-SOF for treatment of chronic HCV in Upper Egypt so we performed this study that aimed to determine the efficacy of Simeprevir-Sofosbuvir for treatment of chronic HCV infection among patients attending Sohag Cardiac and Digestive center (Ministry of Health) and explore the safety profile of Simeprevir-Sofosbuvir during treatment of chronic HCV infection. The current study included 100 patients who were classified into 66 patients with chronic hepatitis C (CH Group) and 34 patients with liver cirrhosis (LC Group). HCV RNA by PCR was negative in 99% of our patients at 4 weeks, was negative in 94% at 12 weeks. Sustained virological response (SVR) (virological response 12 weeks after end of treatment) was maintained in 89% of them. Patients with chronic hepatitis showed significantly higher ETVR (97%) and SVR (94%) in comparison to those with LC (88.2%) and (79.4 %) respectively (P value < 0.001)
Similarly, Sulkowski et al [6]. showed that the combination of SOF+SMV achieved an SVR12 rate of 87.4%, 87.9% for patients treated with RBV and 87% for patients treated without RBV. Those results showed that the combination of SOF+SMV is efficacious in HCV GT4 patients with advanced fibrosis. They did not observe a significant difference in SVR12 between patients with severe fibrosis F3 and patients with cirrhosis.
The current study results were comparable to the results of the OPTIMIST-2 study in GT1 patients with cirrhosis treated with SOF + SMV, who achieved SVR12 of 83% [7].
Lower SVR rates for cirrhotics in a real-world setting were also reported by the national Viral Hepatitis (VA) experience (70.0%)[8] and HCV-TARGET (Hepatitis C Therapeutic Registry and Research Network (80.5%) [6]. However, the national VA report was only able to identify patients as cirrhotics based upon the FIB-4 and aspartate aminotransferase to platelet ratio index tests [8]. In contrast, one strength of Sulkowski et al [6]. study was the presence of more complete and definitive clinical data to identify cirrhotic patients. Our treatment outcomes were more in line with those reported by HCV-TARGET [6].
In study of Sclair et al. (2016) [9], twelve weeks of therapy with sofosbuvir and Simeprevir in patients with compensated cirrhosis was inadequate, and explaining why all 10 treatment failures had cirrhosis. Nineteen of the 59 cirrhotic patients were treated with weight-based ribavirin and relapse occurred in 2 of them. Fourteen cirrhotic patients were not treated with ribavirin and relapse occurred in 8 of them, which suggests that the addition of ribavirin may have improved outcomes in cirrhotic patients. Currently, 24 weeks of therapy is recommended by the Infectious Disease Society of America and the American Association for the Study of Liver Diseases for better SVR outcomes in cirrhotic patients.
A large study from Egypt by El-Khayat et al. (2016) [10] done on 583 patients (335 males) showed that the SVR12 was 89.9% and 89.1% among patients with mild fibrosis (F1) and (F2), respectively. The SVR was 97.7% among F3 cases and only 80.8% among F4 patients.
8. CONCLUSION
In conclusion, we found in this study of patients with chronic hepatitis C and HCV related cirrhosis that the combination of SMV-SOF is efficacious and well tolerated. This combination seems to be a good therapeutic option for treatment of chronic hepatitis C infection. Patients with chronic hepatitis C showed significantly higher ETVR and SVR in comparison to those with LC (97% and 94% versus 88.2% and 79.4%) respectively. The total occurrence of side effects among our study population was 13%, and all of them were mild.
References
- Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology; 61:77–87(2015).
- Ray SC, Arthur RR, Carella A, Bukh J, Thomas DL.. Genetic epidemiology of hepatitis C virus throughout Egypt. J Infect Dis.; 182(3):698-707 (2000).
- Casado JL, Mena A, Banon S, Moreno A, Castro A, Perez-Elias MJ, et al.. Efficacy and safety of etravirine-containing regimens in a large cohort of HIV/HCV co infected patients according to liver fibrosis J Int AIDS Soc.;17(4 Suppl 3):19574(2014).
- Papastergiou V, Tsochatzis EA. Letter: scoring models in alcoholic hepatitis - authors' reply Aliment Pharmacol Ther.; 42(1):127(2015).
- Yau AH, Yoshida EM. Hepatitis C drugs: the end of the pegylated interferon era and the emergence of all-oral interferon-free antiviral regimens: a concise review Can J Gastroenterol Hepatol.; 28(8):445-51(2014).
- Sulkowski MS, Vargas HE, Di Bisceglie AM, Kuo A, Reddy KR, Lim JK, et al. Effectiveness of Simeprevir Plus Sofosbuvir, With or Without Ribavirin, in Real-World Patients With HCV Genotype 1 Infection Gastroenterology; 150(2):419-29(2016).
- Lawitz E, Matusow G, DeJesus E, Yoshida EM, Felizarta F, Ghalib R, et al. Simeprevir plus sofosbuvir in patients with chronic hepatitis C virus genotype 1 infection and cirrhosis: A phase 3 study (OPTI-MIST-2) Hepatology; 64(2):360-9(2016).
- Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Effectiveness of sofosbuvir-based regimens in genotype 1 and 2 hepatitis C virus infection in 4026 U.S. Veterans Aliment Pharmacol Ther.; 42(5):559-73(2015).
- Sclair SN, Hernandez MD, Vance E, Gilinski D, et al: Sofosbuvir and Simeprevir Combination Therapy for HCV Genotype 1 Infection: Results of a Single-Center VA Experience. Gastroenterol Hepatol (N Y); 12(8):490-7(2016).
- El-Khayat HR, Fouad YM, Maher M, El-Amin H, Muhammed H. Efficacy and safety of sofosbuvir plus simeprevir therapy in Egyptian patients with chronic hepatitis C: a real-world experience Gut. 66 (11):2008-2012(2016). doi: 10.1136/gutjnl-2016-312012.