Information
Journal Policies
A Case Report of a Patient with Gluten Enteropathy: The Importance of Immunological Markers for Diagnosis and Followup
Tsvetelina Velikova1*, Gergana Taneva2,3, Stanislav Chourchev3, Lyudmila Tankova3, Borislav Vladimirov3
2.Clinic of Gastroenterology, Uni Hospital, Panagyurishte
3.Clinic of Gastroenterology, University Hospital "Tsaritsa Yoana - ISUL" - Sofia, Medical University - Sofia
Copyright : © 2018 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Gluten enteropathy, or celiac disease (CD), is an autoimmune disease of the small intestines in genetically predisposed children and adults caused by immunological hypersensitivity to gluten-containing food. The gold standard for the diagnosing CD and/or gluten-related hypersensitivity is an endoscopic study with a series of multiple colon biopsies (from duodenum) and serological confirmatory methods, including antibodies to tissue transglutaminase - tTG (anti-tTG), antibodies against deamidated gliadin peptides (anti-DGP), anti-gliadin antibodies (AGA), etc.
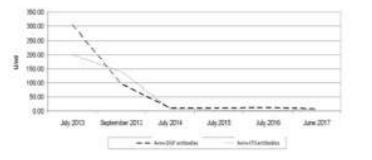
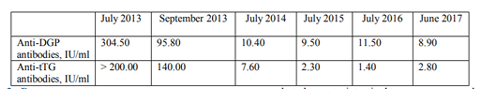
Description of the clinical case: We present a 40-year-old Caucasian woman diagnosed with CD in early age, on a gluten-free diet (GFD) by the age of seven, then switched to a common diet. Since 2006 the patient complained of anemia and frequent diarrheal episodes. She admitted to the hospital with exacerbated gastrointestinal symptoms and swelling of the limbs. The diagnosis of CD is confirmed histologically (partial atrophy of the villous apparatus, increased number of inter- and intraepithelial lymphocytes) and serologically with immunological markers (anti-tTG antibodies IgA+IgG (>200 U/ml, URL < 15 U/ml), anti-DGP IgA+IgG+IgM (304.5 U/ml, URL < 15 U/ml), without IgA deficiency (serum IgA in norm). Following compliance with a GFD, the patient has improved their condition and a decrease of the antibodies levels was observed (anti-tTG 140.0 U/ml, anti-DGP 95.8 U/ml).
Conclusion: The celiac-specific autoantibodies have decreased, indicating a response from GFD; however, they stayed above the upper reference limit. Along with clinical, imaging and histology studies, antibody testing helps to diagnose and determines the severity of the disease. Antibodies in the blood can be used for follow-up and measurement of the GFD adherence, and possibly for prognosis of refractory and complications of the disease.
celiac disease, gluten enteropathy, autoantibodies, anti-tTG, anti-DGP, gluten-free diet, follow-up, Gastroenterology
1. Introduction
Gluten enteropathy, a.k.a. celiac or non-tropical sprue is an autoimmune disease of the small intestines which arise in genetically predisposed children and adults[1]. It is thought to be caused by immunological hypersensitivity to gluten-containing food [2]. The key aspects of the pathogenesis of the disease include the enzyme tissue transglutaminase (tTG). Intestinal tTG is a calcium-dependent enzyme that catalyzes hydrolysis (i.e., deamidation, conversion of the amino acid glutamine into glutamate) of glutamine residues. Gliadin, a protein rich in glutamine, is a specific substrate for this enzyme. tTG selectively deamidates the gliadin molecules trapped in lamina propria so that deamidated gliadin peptides are formed [3].
The complex of the gliadin and the tTG and deamidated gliadin peptides in association with HLA-DQ2 (or DQ8 and other) molecules can be more readily represented by gut mucosal T lymphocytes [4]. However, this process is not performed in healthy individuals. Gluten-sensitive CD4+ T cells are activated and a Th1 immune response develops associated with the production of large amounts of IFNγ leading to mucosal damage [4]. Activated Th lymphocytes support the differentiation of specific B-lymphocytes into plasmocytes secreting specific antibodies such as anti-gliadin antibodies (AGA), anti-tTG (anti-tTG), anti-deamidated gliadin peptides (anti-DGP) antibodies, etc. [5].
These antibodies are strongly celiac-related and could be employed not only in the diagnosing but also in managing and follow-up of the disease [5,6].
2. Description Of The Case Report
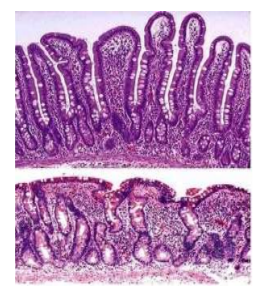
We present a clinical case of a 40-year-old Caucasian woman, diagnosed with CD at the age of 3 years. She was administered to GFD for four years, then discontinued the diet and switched to a common diet. Then, she complained frequently of diarrheal episodes with predominantly spring-autumn character; and a persistent anemia. In 2013, she started to complain of diarrhea every day, with no pathological impurities, swelling of the limbs and pronounced flatulence. She presented at the Gastroenterology Clinic with the above-mentioned symptoms. The present status and imaging were normal. From the laboratory testing, we found iron deficient anemia - Hemoglobin 91 g/l, Iron 4.6 μmo/l. We observed also positive immunological markers in the blood - anti-tTG antibodies IgA+IgG (>200 U/ml, URL < 15 U/ml), anti-DGP IgA+IgG+IgM (304.5 U/ml, URL < 15 U/ml). Serum levels of IgA were within the reference range - 1.76 g/l (reference range: 0.71-3.60 g/l).Histological investigation showed partial atrophy of the villous apparatus and increased number of inter- and intraepithelial lymphocytes, which was congruent with the typical picture of CD[7] (Figure1)
The patient was discharged from the hospital with recommendations for hygienic-dietary regimen and strict adherence to a GFD. The patient was followed-up two months later by retesting for the immunological markers. Anti-tTG antibodies decreased to 140.0 U/ml and anti-DGP dropped to 95.8 U/ml (Figure 2). However, they stayed above the upper reference line. Serum IgA was unchanged - 1.36 g/l (0.71-3.60).
3. Discussion
We presented a case of a 40-year-old female who has been diagnosed with CD in the childhood, but who has not maintained a GFD for about 33 years, with complaints of exacerbated gastrointestinal symptoms and persistent anemic syndrome. Diagnosis of CD was re-confirmed histologically and serologically (immunologically) in elderly. Due to the common presence of IgA deficiency along with CD, the former was excluded. After adherence to GFD and strict compliance of the patient, she has improved her condition and has reduced the antibody levels in two months. Firstly, their levels stayed above the URL, explained by both the persistence of the disease and the relatively short 2-month-period on GFD.
After CD diagnosis, patients need a permanent adherence to a GFD, and they should be monitored periodically and routinely for celiac-related antibodies to assess compliance with the diet [2]. For the follow-up, different celiac-related antibodies could be employed. However, anti-DGP antibodies have been shown to be more sensitive in unveiling compliance to GFD compared to anti-tTG, even though the latter showed the enhanced diagnostic sensitivity for CD [8]. However, in clinical practice, even strong positive anti-tTG IgA results did not necessarily correlate with the severity of villous atrophy, so they are unlikely to be useful for monitoring dietary adherence [9]. It is important to note that all antibody-based serologic tests are expected to normalize on GFD, so the test accuracy depends on ongoing gluten consumption [10]. Furthermore, if the antibodies do not normalize within a couple of months on GFD, the physician should suggest refractory CD. These findings, however, require further investigations. We should also point out that some CD patients may be negative for celiac-related antibodies, which is not our case. The explanations for these CD patients with villous atrophy but without positive auto antibodies are GFD administration at the time of testing, IgA deficiency, non-celiac enteropathy or just seronegative CD [1].
4. Conclusion
Combine testing of antibodies related to CD, a.k.a. a multiplex immunoassay improves patient diagnosis. Moreover, repeated antibody testing can be used for monitoring the effect of the GFD and the clinical course and severity of the disease. Our patient was advised to monitor antibodies at least every 3-12 months, and also to keep under observation the bone density and for neurological manifestations of the disease.
References
- Kaswala DH, Veeraraghavan G, Kelly CP, et Leffler DA. Celiac Disease: Diagnostic Standards and Dilemmas. Diseases 2015; 3:86-101.
- Dias JA. Celiac Disease: What Do We Know in 2017? GE Port J Gastroenterol 2017; 24:275– 278.
- Arslan N. Towards Finding More Diagnostic Serological Markers in Celiac Disease: Can Deamidated Gliadin Peptide Antibodies Help to Our Babies? International Journal of Celiac Disease 2013; 1(1):27-28.
- Mazzarella G. Effector and suppressor T cells in celiac disease. World J Gastroenterol 2015; 21(24): 7349-7356.
- Schyum AC, et Rumessen JJ. Serological testing for celiac disease in adults United European Gastroenterology Journal 2013; 1(5):319–325.
- VelikovaTV, Spassova ZA, Tumangelova-Yuzeir KD, Krasimirova EK, Ivanova-Todorova EI, Kyurkchiev DS, et Altankova IP. Serological Update on Celiac Disease Diagnostics in Adults. International Journal of Celiac Disease 2018; 6(1):20-25.
- Shannahan S, Leffler DA. Diagnosis and Updates in Celiac Disease. Gastrointest Endoscopy Clin N Am 2017; 27:79–92.
- Monzani A, Rapa A, Fonio A, Tognato E, Panigati L, Oderda G. Use of deamidated gliadin peptide antibodies to monitor diet compliance in childhood celiac disease. J pediatr gastroeneterol nutr 2011; 53(1):55-60.
- Lerner A, Jeremias P, Matthias T. Outside of Normal Limits: False Positive/Negative Anti TG2 Autoantibodies. International Journal of Celiac Disease 2015; 3(3):87-90.
- Daniel A. Leffler et Schuppan D. Update on Serologic Testing in Celiac Disease. Am J Gastroenterol 2010; 105:2520–2524