Information
Journal Policies
Rare Occurrence of a Metastatic Diffuse Large B-Cell Lymphoma within a Tubular Adenoma: Case Report and Review of the Literature
Tracey Martin1,Andrea Hernandez2,Adam Goodman3,Renee Williams4
2.New York University Langone Medical Center, Department of Pathology, New York, NY, USA.
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Diffuse large B-cell lymphoma (DLBCL) represents about 90% of all non-Hodgkin’s lymphoma (NHL) and is the most common pathological type of gastrointestinal (GI) lymphomas. The GI tract is the most common extranodal site for lymphomas with primary GI lymphomas being relatively rare. Here we present an unusual incidental finding of DLBCL infiltrating a tubular adenomatous polyp with subsequent evidence of metastatic disease on Positron emission tomography (PET) scan.
Colon, diffuse large B-cell lymphoma, adenoma, Gastroenterology
1. Introduction
Colorectal lymphoma represents less than 0.5 % of primary colorectal neoplasms with diffuse large B-cell lymphoma (DLBCL) being the most common subtype. [1,2,3] To our knowledge, synchronous occurrence of lymphomas within a gastrointestinal polyp has been reported in 5 cases with two of these cases describing DLBCL. [1,4] Here, we present an incidental finding of metastatic DLBCL within a tubular adenoma. While there are few reports of lymphomas in an adenoma, ours is the second reported DLBCL subtype to present with metastatic disease and the only case where chemotherapy was the mainstay of treatment.
2. Case Report
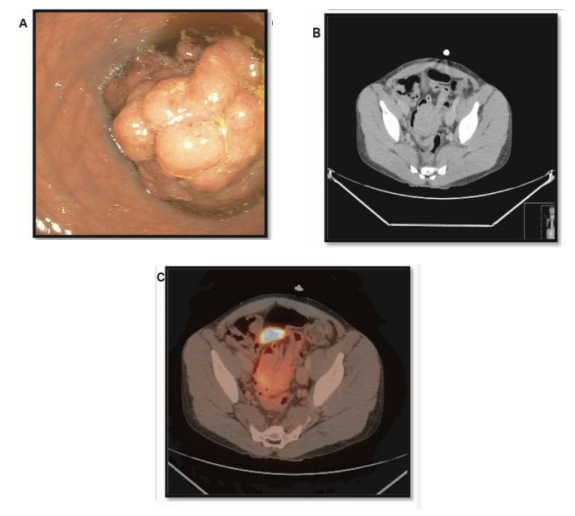
A 31-year-old man with a past medical history of hepatoblastoma treated with resection and adjuvant chemotherapy presented with fevers and back pain. Computed tomography (CT) scan of the abdomen showed pyelonephritis and an incidental finding of sigmoid colo-colonic intussusception. Colonoscopy revealed a 4cm fungating mass occupying 75% to 99% of the colonic lumen, causing a partial obstruction (Figure 1). CT scan of the chest, abdomen and pelvis was without evidence of lymphadenopathy or metastatic disease. Immunohistological evaluation of the biopsied mass was consistent with DLBCL and fragments of tubular adenoma. Positron emission tomography (PET) scan two months later showed mildly hypermetabolic non-enlarged lymph nodes in the neck and axilla bilaterally and attenuations in the upper pole of the right kidney, a right upper pulmonary nodule, and in the sigmoid mass (Figure 1). A bone marrow biopsy showed no evidence of lymphoma The patient was started on chemotherapy and completed one cycle of cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab (R-CHOP), three cycles of cyclophosphamide, doxorubicin, vincristine, prednisone, rituximab and etoposide (EPOCH –R) and one cycle of rituximab, cyclophosphamide, prednisone and etoposide (R-CEOP). Repeat PET scan, showed resolution of cervical and axillary nodes, and the lung opacity suggesting complete response to chemotherapy. However, the colonic mass remained. A sigmoidoscopy again demonstrated a large sigmoid mass; which endoscopically appeared to have decreased in size. Pathology showed a tubular adenoma with no evidence of lymphoma. A post-treatment bone marrow biopsy was negative for lymphomatous involvement. Endoscopic resection of the rectosigmoid mass was unsuccessful and surgical resection was recommended, however the patient was lost to follow up.
3. Pathology
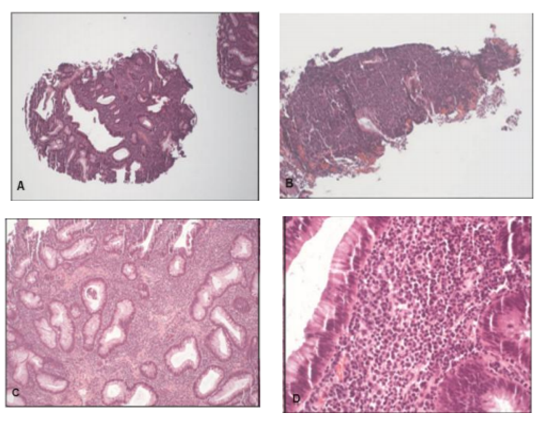
Histologically, the sigmoid mass revealed fragments of tubular adenoma. Characteristic adenomatous mucosal changes were seen (i.e., hyperchromatic, elongated nuclei, loss of mucinous goblet cells). There was a dense cellular infiltrate within the lamina propria effacing the normal colonic wall architecture (Figure 2).
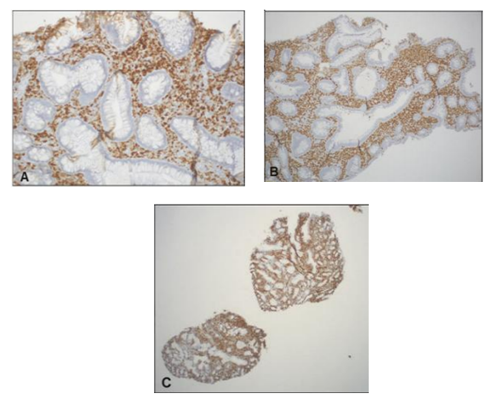
The cellular infiltrate, composed of an atypical, monomorphic lymphoid population was diffusely positive for PAX5 and CD79a (B-cell markers) and negative for CD5, CD10, BCL6 and cyclin D1. Characterization of the atypical B-lymphocytes demonstrated immunoreactivity for MUM-1 and a proliferation index over 95% by Ki-67 (Figure3). Molecular genetic analysis showed immunoglobulin heavy chain gene rearrangements. The morphologic and immunophenotypic features were diagnostic of DLBCL, non-germinal center type with an increased proliferation index.
A subsequent biopsy of the sigmoid mass, following chemotherapy, showed a tubular adenoma with no evidence of lymphoma. In addition, flow cytometry of both the mass and bone marrow aspirates demonstrated no immunophenotypic evidence of NHL.
4. Discussion
The colon accounts for approximately 6-20% of all primary gastrointestinal NHLs, 14-38% originate in the small intestine and 60-75% in the stomach.[5]While DLBCL is the most common sub-type of colorectal lymphoma, the synchronous occurrence of a colorectal lymphoma with a tubular adenoma is extremely rare. [5] Of the 5 reported cases, two were cases of DLBCL. Genovese et al. reported a case of B-cell lymphoma in an adenoma, however, there was no evidence of metastatic disease and no follow up regarding treatment. [4] Similar to this case, Roeb et al. described a case of disseminated DLBCL in a colorectal polyp but surgical resection was the mainstay of treatment without further chemotherapy or a discussion of the clinical status post-treatment. [1] In our case the patient had evidence of metastatic disease treated with chemotherapy and although the colonic mass remained, there was no further evidence of lymphoma based on PET imaging and pathologic evaluation.
Most authors classify primary GI lymphomas as arising in any part of the GI tract even in the presence of disseminated disease as long as the extranodal site is predominant.[3] Symptoms are non-specific but can include abdominal pain, altered bowel habits, weight loss, and lower gastrointestinal bleeding. [2] The gold standard diagnostic modality includes colonoscopy with biopsy. [3] CT scan and other imaging modalities can be helpful in evaluation for metastatic disease.
Histologic examination will show a diffuse neoplastic proliferation of large to medium-sized lymphoid cells. Phenotypically, these tumor cells are positive for B cell markers including CD20, CD 22, PAX-5 and CD79a.[3] CD 10, a marker for follicular derived DBCL, is expressed in 30-60% of cases, with BCL-6 expressed in 70% of cases and MUM1/IRF4 in 35% to 65%. [3] Colorectal DLBCL is often treated with chemotherapy, sometimes in conjunction with surgical resection and or radiation. CHOP has been the standard of care since 1970s. [6] However, R-CHOP is now widely used as studies have demonstrated improved complete response with an increase in survival rates by 10-15% in comparison to CHOP alone. [3,7] In 2002, Wilson et al designed a regimen, EPOCH-R which has subsequently been shown by various studies to have good long-term outcome in poor prognosis DLBCL. [8]
Though colonic resection of the mass is often recommended, especially in cases with limited disease, there are no randomized trials to support surgical intervention as colonic lymphoma is quite rare. [3,9] Radiotherapy is sometimes used pre-operatively or in conjunction with chemotherapy for DBCL, however given risk of colonic complications radiotherapy may be limited in this cohort.[10]
Although unusual, the diagnosis and management of colonic lymphoma is challenging.
The correlation of clinicopathologic findings is critical, particularly in lymphomas that harbor within an adenomatous lesion. This case report helps to alert physicians of this rare finding and helps to support its inclusion in the differential diagnosis for polypoid lesions in the gastrointestinal tract.
References
- Roeb E., Rummel M., Blau W., et al. B-cell lymphoma in a tubular adenoma with high-grade dysplasia: a rare extramedullary manifestation of high-grade diffuse large B-cell lymphoma. Endoscopy. 43(S02), (2011).
- Ghimire P., Wu G.Y. and Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 17(6), 697-707, 2011.
- Barbaryan A., Ali A. M., Kwatra S.G., et al. Primary diffuse large B-cell lymphoma of the ascending colon. Rare Tumors. 5(2), 85-88, 2013.
- Genovese F., Becchina G., Nagar C., et al. Primary diffuse large B-cell lymphoma developing within a rectal tubular adenoma with low-grade dysplasia: a case report. J Med Case Rep. 8(1), 103, 2014.
- Kam J.C., Gauchan D., Doraiswamy V., et al. Follicular lymphoma presenting as an isolated colonic polyp. J Gastrointest Cancer. 45(3), 383-386, 2014.
- Armitage J.O. My treatment approach to patients with diffuse large B-cell lymphoma. Mayo Clin Proc. 87(2), 161-171, 2012.
- Purroy N., Bergua J., Gallur L., et al. Long-term follow-up of dose-adjusted EPOCH plus rituximab (DA-EPOCH-R) in untreated patients with poor prognosis large B-cell lymphoma. A phase II study conducted by the Spanish PETHEMA Group. Br J Haematol. 169(2), 188-198, 2015.
- Wilson W.H., Grossbard M.L., Pittaluga S., et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 99(8), 2685-2693, 2002.
- Stanojevic G.Z., Nestorovic M.D., Brankovic B.R., et al. Primary colorectal lymphoma: An overview. World J Gastrointest Oncol. 3(1), 14-18, 2011.
- Aleman B.M., Haas R.L. and van der Maazen R.W. Role of radiotherapy in the treatment of lymphomas of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 24(1), 27-34, 2010.