Information
Journal Policies
Evaluation of Aspartate Aminotransferase Platelets Ratio Index (APRI) and FIB-4 as Non Invasive Predictors of Hepatic Fibrosis in Patients with Chronic Hepatitis C
Khairy H Morsy1,Mahmoud Saif -Al-Islam2,Amr A Hamed3
Copyright : © 2017 . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Objectives: This study aims to assess simple models from routine investigation for prediction of significant fibrosis (Ishak score > 2) and cirrhosis in CHC patients.
Patients and Method: Our retrospective cohort study studied 100 chronic HCV patients that had liver biopsy. Two indices were assessed, aspartate aminotransferase platelets ratio index (APRI) and FIB-4 to predict significant fibrosis and cirrhosis.
Result: APRI was accurate in prediction of significant fibrosis and cirrhosis, with area under ROC: 0.74 and 0.84 respectively. Using one single cut-off, we could predict absence or presence of significant fibrosis in 69.9% and 80.8% of patients respectively. The FIB-4 had higher accuracy in prediction of significant fibrosis and cirrhosis, with area under ROC: 0.75 and 0.85 respectively. Using one single cut-off, we could predict absence or presence of significant fibrosis in 71% and 81% of patients respectively.
Conclusion:This study demonstrated that we can predict significant fibrosis and cirrhosis in patients with CHC by simple indices using available investigations with a high degree of accuracy. Using APRI and FIB-4 may decrease the need for liver biopsy in CHC patients.
APRI, FIB-4, fibrosis, HCV infection,Hepatology, Gastroenterology
1. Introduction
Hepatitis C virus (HCV) infection is considered an important cause of hepatic diseases in the world [1]. More than 200 million persons are infected with HCV in the world [2]. About 15% of the Egyptians had positive HCV antibodies, while 10 % had HCV viraemia [3]. HCV is one of the important causes of chronic hepatitis, liver cirrhosis, hepatocellular carcinoma (HCC) and liver transplant in Egypt [4].
Liver biopsy examination is important to evaluate chronic HCV patients [5]. Patients who have significant fibrosis develop almost invariably cirrhosis in 10-20 years so HCV treatment is important [6]. Liver biopsy seems to be a safe intervention but it costs much and has risk for complication [7].
Recently many noninvasive markers (NIMs) for assessing liver fibrosis have been developed, and they are frequently used in clinical practice [8]. Noninvasive predictors for assessment of histology in CHC patients include clinical picture, routine investigations, serum fibrosis or inflammation markers, and imaging [9,10].
The APRI is acceptably accurate for liver fibrosis assessment in CHC patients, but not in those with chronic HBV [11]. The FIB-4 score was mainly used in HIV-HCV co-infected patients. Vallet- Pichard validated this model in a cohort study of patients with HCV mono-infection [12].
This study aimed to assess simple models from routine investigations for significant fibrosis (Ishak score > 2) and cirrhosis prediction in HCV patients.
2. Patients And Method
Our retrospective study studied 100 CHC patients that underwent liver biopsy in the Tropical Medicine and Gastroenterology Department, Sohag University Hospital, Egypt.
Chronic HCV patients diagnosed by (PCR) and liver biopsy.
1. Other causes of liver disease.
2. Liver malignancy.
3. Previous liver transplant.
4. Previous interferon or immunosuppressive therapy.
A list of 100 chronic HCV patients who underwent percutaneus liver biopsy in Tropical Medicine Department was obtained from our medical records. Clinical examination, laboratory investigations data about those patients were obtained. We used laboratory data done within 4 weeks from the liver biopsy date. APRI score according to Wai’s formula [13]:
We used Ishak score [15] as scoring technique:
F0: No fibrosis
F1: Fibrous expansion of some portal areas, with or without short fibrous septa
F2: Fibrous expansion of most portal areas, with or without short fibrous septa
F3: Fibrous expansion of most portal areas with occasional portal to portal (P-P) bridging
F4: Fibrous expansion of portal areas with marked bridging [portal to portal (P-P) as well as portal to central (P-C)]
F5: Marked bridging (P-P and/or P-C) with occasional nodules (incomplete cirrhosis)
F6: Cirrhosis, probable or definite We considered stage 0-2 non-significant fibrosis, and stage 3-6 significant fibrosis. We compared APRI and FIB-4 with liver biopsy to evaluate the possibility of using them as non-invasive methods to predict significant fibrosis and cirrhosis.
We got acceptance of Ethical Committee. Patients signed informed written consent before collecting the data.
Data were represented as mean, mean standard error, and range. Data were analyzed using student Mann-Whitney to compare two groups as the data were not normally distributed. Qualitative data were presented as numbers and percentages and compared using Chi square test. Data analysis was by sensitivity, specificity, positive, and negative predictive value according to receiver operating characteristic (ROC) curve. We use multivariate analysis to identify predictors of fibrosis and cirrhosis. P value was considered significant if it was less than 0.05.
3. Results
The mean patients age was 41.16 ± 1.19 years, 68 (68%) were men. 45% patients were with significant fibrosis and 15% were with cirrhosis (Table 1).
We used univariate analysis for assessment of factors with significant fibrosis and cirrhosis (Table 2).
Multivariate analysis demonstrated that age (P=0.03), ALT level (P=0.04), AST level (P=0.002), and platelet number (P=0.04) were the independent significant fibrosis predictors while AST level (P=0.02), and platelets number (P=0.02) were nondependent predictors of cirrhosis (Table 3).
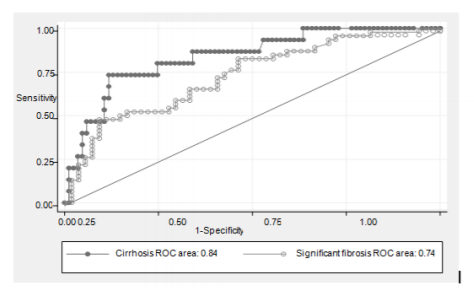
ROC curves for significant fibrosis prediction with APRI with AUC of 0.74 and cirrhosis with AUC of 0.84 were plotted in Figure 1.
According to the ROC, we chose 2 cut-off points for prediction the presence (coordinate B: APRI < 0.84) or absence (coordinate A: APRI ≤ 0.21) of significant fibrosis. Patients had APRI greater than 0.84, 10 from 12 (83.3%) would be with significant fibrosis. Patients had APRI = 0.21 or less, 15 of 17 (88%) did not have significant fibrosis. The best cut off point is at 0.58 with accuracy 69.9% (Table 4).
We also chose 2 cut-off points for prediction the presence (coordinate D: APRI < 1.05) or absence (coordinate C: APRI ≤ 0.27) of cirrhosis. Patients had APRI greater than 1.05, 4 of 7 (57.1%) had cirrhosis. Patients had APRI of 0.27 or less, 31 of 31 (100%) would not have cirrhosis, and 3 only of 85 (3.5%) without cirrhosis would be identified falsely with accuracy 61.6%. The best cut off point is at 0.66 with accuracy 80.8% (Table 5).
According to the ROC, we chose 2 cut-off points for prediction the absence (coordinate A: FIB-4≤0.64) or presence (coordinate B: FIB-4 < 1.76) of significant fibrosis. Patients had FIB-4 of 0.64 or less, 15 of 17 (88%) did not have significant fibrosis. In patients having FIB-4 greater than 1.76, 19 of 21 (90.5%) would have significant fibrosis. The best cut off point is at 1.44 with accuracy 71 % (Table 6).
APRI is < 0.84 (the greater cut-off level for significant fibrosis), hence the positive predictive value for significant fibrosis is 83.3%. The APRI value is > 1.05 (the greater cut-off value for cirrhosis), hence the negative predictive value for cirrhosis is 88%. So, these patients may have significant fibrosis not cirrhosis.
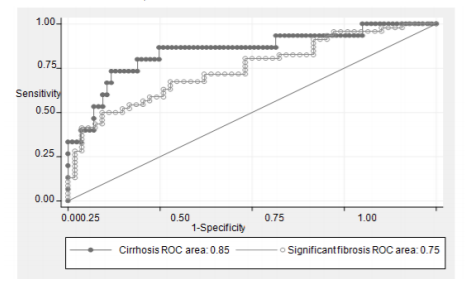
ROC curves to predict significant fibrosis and cirrhosis with FIB-4 were in Figure 2 with AUC 0.75 and 0.85, respectively.
We also chose 2 cut-off points for prediction the presence (coordinate D: FIB-4 < 2.54) or absence (coordinate C: FIB-4 ≤ 0.84) of cirrhosis. Patients had FIB-4 more than 2.54, 5 of 5 (100%) had cirrhosis. On the other hand, for patients with FIB-4 of 0.84 or less, 37 of 38 (97%) would not have cirrhosis. The best cut off point is at 1.29 with accuracy 81% (Table 7).
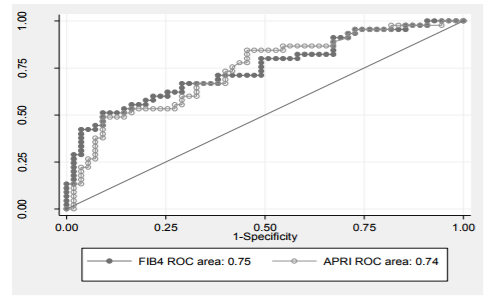
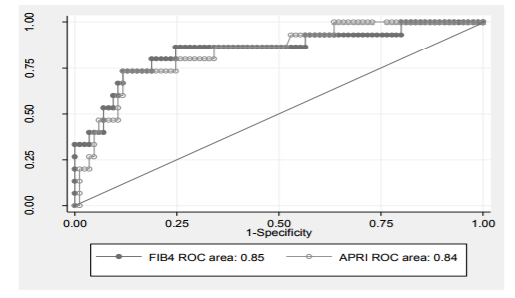
FIB-4 is < 1.76 (the greater cut-off level for significant fibrosis), hence the positive predictive value for significant fibrosis is 90.5%. FIB-4 is > 2.54 (the greater cut-off value for cirrhosis), hence the negative predictive value for cirrhosis is 89.5%. So, this patient may have significant fibrosis not cirrhosis. The FIB-4 score showed better AUROC curve (0.75, 0.85) than APRI score (0.74, 0, 84) regarding significant fibrosis and cirrhosis prediction (Figures 3, 4).
4. Discussion
Our study tried to evaluate one or more scores with the help of routine investigations for prediction cirrhosis and significant fibrosis in CHC patients. This study demonstrated that platelets number and AST level were the independent predictors for cirrhosis, while age, platelets number, ALT and AST levels were the independent predictors for significant fibrosis. Our results were like the results of many studies before, that platelets number and AST level were significant for prediction of either cirrhosis or significant fibrosis [16-18].
To detect the relationship between AST level and platelets number and the fibrosis stage, we tested 2 indices, the APRI and FIB-4, which were simple and are comparably accurate with models with 3 or more variables for prediction significant fibrosis and cirrhosis. APRI and FIB-4 in significant fibrosis and cirrhosis prediction were evaluated in a subsequent set of patients with near accuracy. There were many studies to predict of significant fibrosis and cirrhosis in chronic HCV patients in the years before [9, 10, 19].
This study tries to present new features. First, we got a good percentage of patients who had significant fibrosis (45%) and cirrhosis (15%). Secondly, we included patients who had liver biopsies at our center not only patients enrolled in treatment trials [17, 18]. Thirdly, this study studied only patients who did not receive treatment as many previous results showed that liver histology may be better even in non responders to interferon treatment [20, 21]. Last but not least, our predictive score needs objective and easy investigations. Platelets number and AST level are routine investigations for patients with chronic HCV, so no need for additional tests. Many studies reported decreased platelets number and increased AST level with liver fibrosis progression. There is more platelets sequestration with splenomegaly with progression of fibrosis and portal hypertension[22] . Studies in patients with liver transplantation demonstrated that liver fibrosis progression makes hepatocytes produce less thrombopoietin, and so less platelets formation [23, 24].
Progressing liver fibrosis may decrease AST clearance [25], which leads to AST level increase. Also, advanced liver disease may be accompanied with injury of mitochondria, leading to more AST release relative to ALT [26, 27].
We use the APRI score for the difference between AST and platelets values in patients with different fibrosis stages. In Williams and Hoofnagle study [28], it was observed that with progression of chronic liver disease, increase in AST is greater than that in ALT. The investigators exploited the difference between the two factors and the AST/ALT ratio was advised to predict cirrhosis. The value of AST/ALT ratio was confirmed in cirrhosis prediction [29, 30] but there was wide variation in its accuracy, with negative predictive values variation between 72% and 88%, and positive predictive values variation between 64% and 100%. AST/ALT ratio was an independent cirrhosis predictor but it was not sufficient alone for accurate cirrhosis prediction. And also, AST/ALT ratio was not useful alone for significant fibrosis prediction [16, 17].
The APRI was with high accuracy for prediction of significant fibrosis and cirrhosis, with area under ROC 0.74 and 0.84 respectively. Using single cut-off, we can predict absence or presence of significant fibrosis and cirrhosis in 69.9% and 80.8% of patients respectively. Our results were like recent studies presenting APRI score as an important fibrosis and cirrhosis predictor [31, 32]. In predicting significant fibrosis the PPV of the APRI was better than NPV, like results in the study of Wai et al. [13]. NPV of APRI was more than PPV in the cirrhosis prediction, with a higher specificity and a lower sensitivity. In our results, the AUROC of APRI in significant fibrosis prediction was more than that in the study of Cheung et al. [33]. Unlike Silva et al. results, where the AUROC of APRI was 0.92 in significant fibrosis and cirrhosis prediction, the APRI was better in cirrhosis prediction than significant fibrosis [34].
Sterling et al.’s study described FIB-4, consisting of ALT and AST level, platelets number and age, for fibrosis assessment in HIV/HCV co-infected patients [14]. FIB-4 index also was used as an accurate and inexpensive fibrosis marker in HCV mono-infection [12]. The FIB-4 was more accurate in significant fibrosis and cirrhosis prediction, with area under ROC 0.75 and 0.85 respectively. Using single cut-off, absence or presence of significant fibrosis and cirrhosis could be predicted in 71% and 81% of patients respectively. This is like other studies [31, 32]. Recently, Holmberg et al. found that FIB-4 was more accurate than APRI and much more accurate than a simple AST/ALT ratio for predicting advanced fibrosis in CHC patients [35].
Although our study was retrospective, we tried to maximize our data accuracy. To make sure of data extraction consistency, we established predetermined criteria for all subjective variables before records reviewing, and all data was extracted by one researcher. The main variables in this study were objective investigations that were available in the hospital computer system.
We had difficulties in this study. This study included 100 patients admitted in Tropical Medicine Department, Sohag University Hospital, 50% of them were with significant fibrosis on histopathological examination and no one had previous antiviral therapy. Whether we can generalize this results to community-based practice in which patients are with milder disease, or with previous antiviral treatment failure is still to be determined. Last but not least, this study depends on liver biopsy for liver fibrosis assessment, but sampling error and intra- and interobserver variability may affect the correlations between histopathology and noninvasive markers of liver fibrosis.
5. Conclusion
We found that APRI and FIB-4 scores can be used for significant fibrosis and cirrhosis prediction in HCV patients with no previous treatment with a great degree of accuracy. We can use APRI and FIB-4 scores in the clinic or at the bedside. We can avoid liver biopsies by one simple model for predicting significant fibrosis and cirrhosis with good accuracy in treatment-naive HCV patients.
We need more prospective studies for validating APRI and FIB-4 scores in more patients with chronic HCV in other hospitals, especially, community-based practices where there may be lower significant fibrosis and cirrhosis prevalence, and among patients with previous antiviral treatment.
References
- Lavanchy D. The global burden of hepatitis C. Liver Int.; 29:74-81 (2009).
- EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol.; 55(2):245-64 (2011).
- El-Zanaty F, Way A: Egypt Demographic and Health Survey (2008). Egyptian: Ministry of Health. Cairo: El-Zanaty and Associates, and Macro International; (2009).
- El-Zanaty FH, Way AA, al-Sihhah wa-al EW, Macro O. Egypt demographic and health survey, (2005). Ministry of Health and Population (2006).
- Gebo KA, Herlong HF, Torbenson MS, Jenckes MW, Chander G, Ghanem KG, El-Kamary SS, et al. Role of liver biopsy in management of chronic hepatitis C: a systematic review. HEPATOLOGY; 36: S161-S172 (2002).
- Yano M, Kumada H, Hage M, Ikeda K, Shimamatsu K, Inoue O, Hashimoto E, et al. The long-term pathological evolution of chronic hepatitis C. HEPATOLOGY; 23:1334-1340 (1996). HEPATOLOGY, Vol. 38, No. 2, WAI ET AL. 525 (2003).
- Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. HEPATOLOGY; 32:477-481 (2000).
- Castera L, Le Bail B, Roudot-Thoraval F, et al. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol ; 50:59-68 (2009).
- Guechot J, Laudat A, Loria A, Serfaty L, Poupon R, Giboudeau J. Diag-nostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liverfibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin Chem; 42:558-563 (1996).
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T, for the MULTIVIRC group. Biochemical markers of liverfibrosis in patients with hepatitis C virus infection: a prospective study. Lancet; 357:1069-1075 (2001).
- Yilmaz Y, Yonal O, Kurt R, Bayrak M, Aktas B, Ozdogan O. Noninvasive assessment of liver fibrosis with the aspartate transaminase to platelet ratio index (APRI): Usefulness in patients with chronic liver disease. Hepat Mon.; 11(2):103-7 (2011).
- Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S: FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology, 46(1):32-36 (2007).
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C.Hepatology; 38:518-526 (2003).
- Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M, APRICOT Clinical Investigators:Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology, 43:1317-1325 (2006).
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, et al. Histological grading and staging of chronic hepatitis. J Hepato, 22(6):696-9 (1995).
- Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol; 92:1302-1304 (1997).
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients withchronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet; 349(9055):825 (1997).
- Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum amino transferase levels and platelet counts as predictors of degree offibro-sis in chronic hepatitis C virus infection. Am J Gastroenterol; 96: 3142-3146 (2001).
- McHutchison JG, Blatt LM, de Medina M, Craig JR, Conrad A, Schiff ER, Tong MJ. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus In-terferon Study Group. J Gastroenterol Hepatol; 15:945-951 (2000).
- Teuber G, Berg T, Naumann U, Raedle J, Brinkmann S, Hopf U, Zeuzem S. Randomized, placebo-controlled, double-blind trial with interferon-alpha with and without amantadine sulphate in primary interferon-alpha nonresponders with chronic hepatitis C. J Viral Hepat;8:276-283 (2001).
- De Ledinghen V, Trimoulet P, Winnock M, Bernard PH, Bourliere M, Portal I, Remy AJ, et al. Daily or three times per week interferon alpha-2b in combination with ribavirin or interferon alone for the treatment of patients with chronic hepatitis C not responding to previous interferon alone. J Hepatol; 36:819-826 (2002).
- Aster R. Pooling of platelets in the spleen: role inthepathogenesis of “hypersplenic”thrombocytopenia. J Clin Invest; 45:645-657 (1996).
- Kawasaki T, Takeshita A, Souda K, Kobayashi Y, Kikuyama M, Suzuki F, Kageyama F, et al. Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis. Am J Gastroenterol; 94:1918-1922 (1999).
- Adinolfi LE, Giordano MG, Andreana A, Tripodi MF, Utili R, Cesaro G, Ragone E, et al. Hepaticfibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br J Haematol; 113:590-595 (2001).
- Kamimoto Y, Horiuchi S, Tanase S, Morino Y. Plasma clearance of intra-venously injected aspartate aminotransferase isozymes: evidence for pref-erential uptake by sinusoidal liver cells. HEPATOLOGY; 5:367-375 (1985).
- Nalpas B, Vassault A, Le Guillou A, Lesgourgues B, Ferry N, Lacour B Berthelot P. Serum activity of mitochondrial aspartate aminotransferase: a sensitive marker of alcoholism with or without alcoholic hepatitis. HEPA-TOLOGY; 4:893-896 (1984).
- Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Wein-man SA. Mitochondrial injury, oxidative stress, and antioxidant gene ex-pression are induced by hepatitis C virus core protein. Gastro enterology; 122:366-375 (2002).
- Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine amino-transferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology; 95:734-739 (1988).
- Reedy DW, Loo AT, Levine RA. AST/ALT ratio ≥1 is not diagnos-tic of cirrhosis in patients with chronic hepatitis C. Dig Dis Sci; 43:2156-2159 (1998).
- Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastro-enterol; 93:44-48 (1998).
- Martinez SM, Fernandez-Varo G, Gonzalez P, Sampson E, Bruguera M, Navasa M, et al. Assessment of liver fibrosis before and after antiviral therapy by different serum marker panels in patients with chronic hepatitis C.Aliment Pharmacol Ther.; 33(1):138-48 (2011).
- Usluer G, Erben N, Aykin N, Dagli O, Aydogdu O, Barut S, et al. Comparison of non-invasive fibrosis markers and classical liver biopsy in chronic hepatitis C. Eur J Clin Microbiol Infect Dis.;31(8):1873-8 (2012).
- Cheung RC, Currie S, Shen H, et al. Can we predict the degree of fibrosis in chronic hepatitis C patients using routine blood tests in our daily practice? J Clin Gastroenterol; 42: 827-34 (2008).
- Silva RG Jr, Fakhouri R, Nascimento TV, et al. Aspartate aminotransferase-to-platelet ratio index for fibrosis and cirrhosis prediction in chronic hepatitis C patients. Braz J Infect Dis; 12: 15-9 (2008).
- Holmberg SD, Lu M, Rupp LB et al. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clinical Infect Dis; 57(2): 240–246 (2013).