Information
Journal Policies
Thyroid Аbnormalities in Primary Aldosteronism
Joanna Matrozova1*, Roussanka Kovatcheva1, Atanaska Elenkova1, Emil Natchev1, Vladimir Vasilev1, Sabina Zacharieva1
Copyright : © 2019 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: Primary aldosteronism (PA) is the most common form of endocrine hypertension, in which a higher rate of cardiovascular complications has been described. On the other hand the association with thyroid disease has been rarely explored. Aim of the study. To investigate the relationship between PA and thyroid pathology, focusing on nodular goiter, in patients with PA and controls with essential hypertension (EH).
Methods: We investigated 93 patients with PA (40 with aldosterone producing adenoma (APA), 51 with idiopathic hyperaldosteronism (IHA) and 2 undetermined cases). Plasma renin activity (ng/ml/h) and serum aldosterone (pmol/l) were measured by radioimmunoassay. Thyroid ultrasonography was performed using Toshiba ECCOCEE, SSA - 340A and 10 MHz linear transducer.
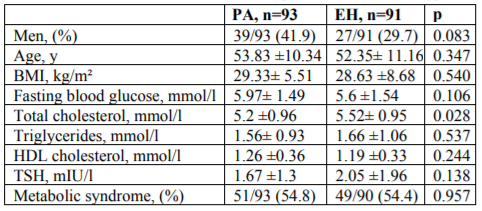
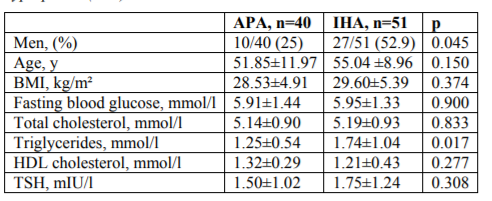
Results: Patients with PA and controls with EH had similar levels of TSH. Patients with EH had significantly higher levels of total cholesterol compared to patients with PA (p=0.028). Over 50% of patients in both groups presented with metabolic syndrome, (54.8% in PA vs 54.4 in EH, p=0.957). There was no statistical significant difference between the prevalence of nodular goiter in PA and EH, although thyroid nodules were found more often in patients with PA (58% vs 47%, p=0.142). There were no significant differences in age, BMI, gender, HDL-cholesterol and fasting blood glucose between APA and IHA. Triglycerides were higher in IHA compared to APA (p=0.017).
Conclusion: Our study reported a high prevalence of nodular goiter in patients with PA and controls with EH. Thyroid nodules were diagnosed more often in patients with PA than in EH although the difference didn't reach statistical significance. Based on the possible relation with the presence of the metabolic syndrome in these patients, screening for thyroid nodules and thyroid dysfunction will be reasonable.
Keywords: primary aldosteronism, nodular goiter, metabolic syndrome, thyroid dysfunction, endocrine hypertension, Diabetes and Endocrinology
1. Introduction
Primary aldosteronism (PA) has been considered a rare cause of hypertension, however recent studies have shown a higher prevalence, ranging between 5-20 % among hypertensive patients [1,2]. Moreover, a higher frequency of cardiovascular and metabolic complications was demonstrated in PA compared to patients with essential hypertension [3,4]. Although PA is the most common form of endocrine hypertension reports of associations between PA and other endocrine pathologies are scarce. The association between PA and diabetes is the only one extensively studied and it is still an unresolved issue [5,6]. More than 20 years ago Beckers et al described aldosterone-secreting adrenal adenoma as part of multiple endocrine neoplasia type 1 (MEN1) in combination with parathyroid adenoma, a prolactinoma, and a toxic multinodular goiter [7]. Later on several other cases of MEN1 including PA as a component were described [8-10]. Recently, we published a case of a female acromegalic patient harbouring Conn's adenoma[11].
On the other hand only a few studies have explored the association between PA and thyroid disease, which is one of the most frequent endocrine disorders. Therefore, the aim of our study was to investigate the relationship between PA and thyroid pathology, focusing on nodular goiter, in a group of patients with PA and controls with essential hypertension (EH).
2. Patients And Methods
The study population consisted of 93 patients with PA (40 with aldosterone producing adenoma (APA), 51 with idiopathic hyperaldosteronism (IHA) and 2 undetermined cases). We made a retrospective analysis of our database and we investigated cases, referred to the Clinical Centre of Endocrinology in Sofia between 2002-2016. Patients are referred to this centre for investigation of endocrine causes of hypertension. The diagnosis of PA was established using the following algorithm: medications that can interfere with aldosterone and renin measurements (beta-blockers, diuretics, angiotensin II receptor blockers, angiotensin-converting enzyme inhibitors) were discontinued for at least 7-10 days, and spironolactone was stopped for at least 45 days prior to blood sampling. Centrally acting medicaments (Moxonodine, Rilmenidine), alpha-blockers, calcium channel blockers, were used for treating hypertension. Blood samples for plasma renin activity (PRA) and aldosterone were taken in the morning between 8-10 h a.m., after the patient had been in sitting position for 30 minutes. The aldosterone-to-renin (ARR) was then calculated and in patients with high ARR (above 750 pmol/l per ng/ml/h) and aldosterone above 416 pmol/l a confirmatory Captopril test was performed. The diagnosis of PA was considered if the aldosterone was> 330 pmol/l after the oral administration of 50 mg of Captopril, while the patient has been sitting for 90 minutes [12]. In all hypertensive subjects other secondary forms of hypertension except PA were ruled out by means of complete medical history, physical examination, and appropriate biochemical and hormonal tests and imaging studies. Among them 91 controls with EH had been selected. The presence of metabolic syndrome was estimated according to the definition of the International Diabetes Federation. All subjects were studied after signing a written informed consent form according to a protocol approved by the Ethics Committee of the University Hospital of Endocrinology, Sofia.
Plasma renin activity (ng/ml/h) was determined by quantitative determination of angiotensin I using a commercially available radioimmunoassay (DiaSorin S.p.A., Saluggia (VC), Italy). Serum aldosterone (pmol/l) was measured by radioimmunoassay (Immunotech, Beckman Coulter Company, Marseille, France). The analytical sensitivity of the method was 0.20 ng/ml, inter-assay CV 7.5%, respectively and intra-assay CV 5.4%.
The cross reactivity with heptapeptide, angiotensin II, and hexapeptide was below 0.02%. We elaborated our own reference range for PRA in sitting position (0.3-3.5 ng/ml/h) [12]. In cases when PRA was < 0.3 ng/ml/h or undetectable it was set at 0.3 ng/ml/h. Serum glucose was measured using enzymatic reference method with hexokinase (Cobas Integra, Roche diagnostics). Serum TSH was measured by immunoradiometric assay (THERMO scientific/BRAHMS, Germany), with reference ranges of 0.3-4.0 mIU/l. The sensitivity was 0.02 mIU/l, with intra- and inter-assay coefficients of variation of 2.5% and 4.1% respectively.
The inter-assay (QC Low and QC High) was calculated, using mean analytic value of all plates according to the formula: CV (%) = standard deviation / mean value of analytic * 100, which corresponds to IFCC protocol. According to generally accepted quality criteria cited above, CV% for interand intra-assay was acceptable, which ensured the reliability of the results.
In all participants thyroid ultrasonography was performed by the same physician, with Toshiba ECCOCEE, SSA - 340A and 10 MHz linear transducer. Thyroid volume was calculated as a sum of both lobes and the isthmus, using the formula for ellipsoid (π/6. a.b.c), where a is the longitudinal, b is the transversal and c is the antero-posterior diameter. The presence of nodule/s >5mm/diameter was determined as nodular goiter.
3. Statistical Analysis
Statistical analysis was performed using SPSS 20.0 for Windows (Chicago, Illinois, USA). The level of statistical significance was set at 0.05. Data distribution normality was tested with Kolmogorov-Smirnov test. Gaussian-distributed data are shown as mean ± SD. Student's t-test for independent samples was used for comparing the equality of means for two groups. Mann-Whiney test was used to compare not normally distributed data. Categorical data were compared by X2 test.
4. Results
The baseline demographic and metabolic characteristics of patients with PA and essential hypertension (EH) are shown in Table 1. There were no significant differences in age, gender, fasting blood glucose, triglycerides and HDL cholesterol levels between the two groups. Patients with PA and controls with EH had similar levels of TSH. Patients with EH had significantly higher levels of total cholesterol compared to patients with PA (p=0.028). A tendency of higher BMI and fasting blood glucose was observed in PA patients. Over 50% of patients in both groups presented with metabolic syndrome.
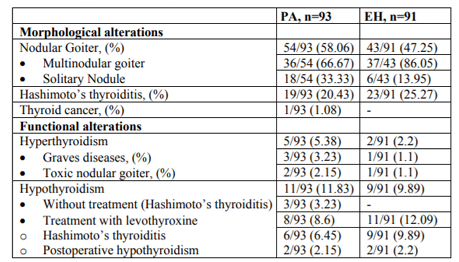
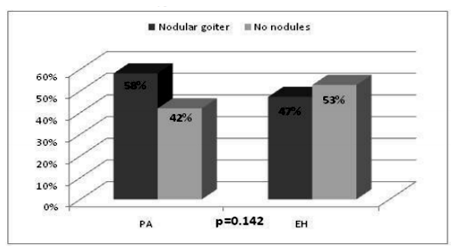
Morphological and functional alterations of thyroid gland in patients with PA and EH are shown in Table 2. There was no statistical significant difference between the prevalence of nodular goiter in PA and EH (Figure.1).
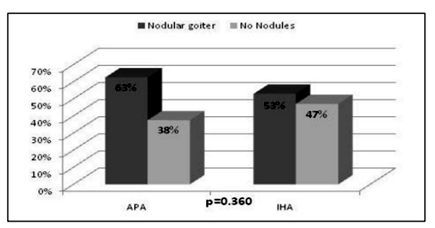
Baseline characteristics of patients with aldosterone-producing adenoma (APA) and idiopathic hyperplasia (IHA) are shown in Table 3. There were no significant differences in age, BMI, gender, HDL-cholesterol and fasting blood glucose between the two groups. Triglycerides were higher in IHA compared to APA (p=0.017). Patients with IHA and APA had similar levels of TSH. Although thyroid nodules were found more often in patients with APA, there was no difference between the prevalence of nodular goiter in IHA and APA (Figure.2).
5. Discussion
Our findings demonstrated high percentage of patients with nodular goiter among the investigated cases (58.06% in patients with PA and 46.74% in patients with EH). This is in accordance with recent findings in the literature which estimate thyroid nodules to be very common in different population settings [13,14]. High-resolution ultrasound series point out a thyroid nodules prevalence of 19-68% among randomly selected individuals and this variability may be due to different factors such as gender, age and iodine intake [15]. Although the prevalence of thyroid nodules is higher in patients with PA compared to EH in our study, it was not statistically significant, which may be due to the relatively small sample sizes. On the other hand, a large population based study, performed among 2415 individuals in Bulgaria in 2006 demonstrated nodular goiter in 567 (23.5%) of the investigated cases [16]. The latter findings along with our results demonstrate a clearly higher prevalence of thyroid nodules in PA compared to the general population. This raises the question of the association of aldosterone and thyroid disorders. There are few clinical and experimental studies that have explored this issue with conflicting results, mainly focusing on the effect of aldosterone on thyroid nodules formation[17,18]. In accordance with our observation, a report by Armanini et al has shown a statistically significant difference in the prevalence of thyroid abnormalities between PA and healthy controls (60% vs 27%, p< 0.0001). In this study the occurrence of multinodular nontoxic goiter was 23.8% in the patients and 6.3% in controls [17]. Another study that investigated the relationship between aldosterone and thyroid disease was performed by Turchi F et al among 188 patients: 92 with PA and 96 matched essential hypertensives (18). The prevalence of thyroid dysfunction was similar in PA and EH (15% and 19%, respectively), although in PA patients a higher prevalence of thyroid nodules was found (66% vs. 46%, P < 0.05). Compared to our results there was a lower prevalence of multinodular goiter (58% vs 47%) which may be related to the lower number of female patients (58% vs 48%), as female gender is known to be associated with thyroid nodules [19]. Another possibility is the lower mean age in Turchi's cohort as older age is related to higher thyroid nodules prevalence [15].
Except for the above-mentioned clinical reports, experimental studies have explored the link between aldosterone and thyroid tissue as well [20-22]. Increased levels of aldosterone have been found in benign colloid nodules as well as aldosterone synthase expression in thyroid tissue [20]. Greenman et al have investigated the possibility for a local production of aldosterone in thyroid tissue [20]. In this study thyroid cyst fluid was investigated in 31 patients and aldosterone levels were found to be elevated above the normal plasma levels in all but five patients. Furthermore, aldosterone synthase mRNA expression was found in aspirates of four of 10 patients. These findings suggest that aldosterone may be locally produced and secreted in thyroid tissue. Other hypotheses discuss that possible imbalance between growth factors (cytokines) and growth inhibitors may be implicated in the pathogenesis of aldosterone overproduction and multinodular nontoxic goiter [21,22]. Also, the role of carbohydrate disorders and insulin resistance has been discussed in the pathogenesis of thyroid nodules formation. Several recent studies demonstrated a higher prevalence of thyroid nodules in patients with metabolic syndrome and reported a correlation between thyroid nodule formation and insulin resistance [23-26]. In view of these findings we can speculate that the high percentage of metabolic syndrome among patients with PA and controls with EH in our study could contribute to the increased prevalence of multinodular goiter. Furthemore, numerous reports have found a higher prevalence of glucose metabolism disorders in PA compared with essential hypertensives or normotensive subjects [5,6]. Thus, it could be hypothesized that the presence of the metabolic syndrome in patients with PA could specifically contribute to the formation of thyroid nodules.
About 20 % of our patients with PA have been diagnosed with autoimmune thyroiditis, which is one of the most common endocrine disorders, especially in women [27,28]. A limited number of studies have investigated the relationship between PA and Hashimoto's thyroditis focusing on the aldosterone proinflammatory effect that could affect the development of autoimmune process [29,30]. Krysiak et al investigated the release of proinflammatory cytokines in a patient with PA, which was increased in the time when thyroiditis coexisted with an APA and was suppressed after surgery of the adenoma. The authors concluded that PA may exacerbate the course of autoimmune thyroid disease and this could be eventually translated to other autoimmune disorders [29].
6. Study Limitations
One of the limits of our study is the retrospective character. However, data were collected during patient care with few missing values. Another limit of the study is the lack of sex and age-matched normotensive control group, although we were able to make reference to data on thyroid pathology from a large population based study, performed in Bulgaria [16].
7. Conclusions
A high prevalence of thyroid nodules was demonstrated in patients with PA and controls with EH. The difference in the prevalence of nodular goiter in patients with PA compared to controls with EH was not statistically significant, probably due to the relatively small sample size. On the other hand, patients with PA had more nodules relative to the Bulgarian general population. We could speculate that presence of the metabolic syndrome in both patients and controls could play a role in the formation of thyroid nodules. Based on these findings, we suggest extensive screening (thyroid function, ultrasound examination) for multinodular goiter in patients with PA.
References
- Ganguly A. Primary aldosteronism. N Engl J Med. 1998; 339: 1828-1834.
- Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Еndocrinol Metab. 2004; 89: 1045-1050.
- Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005; 45: 1243-1248.
- Born-Frontsberg E, Reincke M, Rump LC, Hahner S, Diederich S, Lorenz R, et al. Cardiovascularandcerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism: results of the German Conn's Registry. J Clin Endocrinol Metab. 2009; 94: 1125-1130.
- Hanslik G, Wallaschofski H, Dietz A, Riester A, Reincke M, Allolio B, Lang K, Quack I, Rump LC, Willenberg HS, Beuschlein F, Quinkler M, Hannemann A; participants of the German Conn's Registry. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn's Registry. Eur J Endocrinol. 2015; 173(5):665-75.
- Matrozova J, Steichen O, Amar L, Zacharieva S, Jeunemaitre X, Plouin PF. Fasting plasma glucose and serum lipids in patients with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2009; 53(4):605-10.
- Beckers A, Abs R, Willems PJ, van der Auwera B, Kovacs K, Reznik M, Stevenaert A. Aldosterone-secreting adrenal adenoma as part of multiple endocrine neoplasia type 1 (MEN1): loss of heterozygosity for polymorphic chromosome 11 deoxyribonucleic acid markers, including the MEN1 locus. J Clin Endocrinol Metab.1992; 75(2):564-70.
- Gatta-Cherifi B, Chabre O, Murat A, Niccoli P, Cardot-Bauters C, Rohmer V, Young J, Delemer B, Du Boullay H, Verger MF, Kuhn JM, Sadoul JL, Ruszniewski P, Beckers A, Monsaingeon M, Baudin E, Goudet P, Tabarin A. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d'etude des Tumeurs Endocrines database. Eur J Endocrinol. 2012; 166(2):269-79.
- Kim YL, Jang YW, Kim JT, Sung SA, Lee TS, Lee WM, Kim HJ. A rare case of primary hyperparathyroidism associated with primary aldosteronism, Hürthle cell thyroid cancer and meningioma. J Korean Med Sci. 2012; 27(5):560-4.
- Honda M, Tsukada T, Horiuchi T, Tanaka R, Yamaguchi K, Obara T, Miyakawa H, Yamaji T, Ishibashi M. Primary hyperparathyroidism associatiated with aldosterone-producing adrenocortical adenoma and breast cancer: relation to MEN1 gene. Intern Med. 2004; 43(4):310-4.
- Matrozova J, Vandeva S, Zacharieva S. Case Report: A case report of acromegaly associated with primary aldosteronism. Version 2. F1000Res. 2014; eCollection 2014.
- Matrozova J, Zacharieva S, Kirilov G, Boyanov M. Prevalence of primary aldosteronism among bulgarian hypertensive patients. Central European Journal of Medicine 2010; 5: 399-405.
- Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med 1997; 126: 226–231.
- Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest 2009; 39:699–706.
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26(1):1-133.
- Borissova A.M., Kovatcheva R, Shinkov A, Atanassova I, Vlahov J, Aslanova N, Dakovska L, Vukov M* Endocrine Disorders and Cardiovascular Risk. University hospital of Endocrinology, Medical University – Sofia, Bulgaria. *National Center for Health Information, Medical University – Sofia, Bulagaria. Endocrinologia 2007; 4 (4-18).
- Armanini D, Nacamulli D, Scaroni C, Lumachi F, Selice R, Fiore C, Favia G, Mantero F. High prevalence of thyroid ultrasonographic abnormalities in primary aldosteronism. Endocrine. 2003 Nov; 22(2):155-60.
- Turchi F, Ronconi V, di Tizio V, Boscaro M, Giacchetti G. Blood pressure, thyroid-stimulating hormone, and thyroid disease prevalence in primary aldosteronism and essential hypertension. Am J Hypertens. 2011; 24(12):1274-9.
- Knudsen N, Laurberg P, Perrild H, Bülow I, Ovesen L, Jørgensen T. Risk factors for goiter and thyroid nodules. Thyroid. 2002; 12(10):879-88.
- Greenman Y1, Trostanetsky Y, Ben-Shemen S, Grazas N, Limor R, Osher E, Lewicka S, Vecsei P, Stern N. Thyroid cysts: a new extra-adrenal site of aldosterone synthase expression and increased aldosterone content. Clin Endocrinol (Oxf). 2007; 66(6):886-9.
- Schulte KM1, Antoch G, Ellrichmann M, Finken-Eigen M, Köhrer K, Simon D, Goretzki PE, Röher HD. Regulation of the HGF-receptor c-met in the thyroid gland. Exp Clin Endocrinol Diabetes. 1998; 106(4):310-8.
- Thompson SD1, Franklyn JA, Watkinson JC, Verhaeg JM, Sheppard MC, Eggo MC. Fibroblast growth factors 1 and 2 and fibroblast growth factor receptor 1 are elevated in thyroid hyperplasia. J Clin Endocrinol Metab. 1998; 83(4):1336-41.
- Ayturk S, Gursoy A, Kut A, Anil C, Nar A, Tutuncu NB. Metabolic syndrome and its components are associated with increased thyroid volume and nodule prevalence in a mild-to-moderate iodine-deficient area. Eur J Endocrinol. 2009; 161:599–605.
- Feng S1, Zhang Z2, Xu S3, Mao X3, Feng Y4, Zhu Y2, Liu C3. The Prevalence of Thyroid Nodules and Their Association with Metabolic Syndrome Risk Factors in a Moderate Iodine Intake Area. Metab Syndr Relat Disord. 2017; 15(2):93-97.
- Moon JH, Hyun MK, Lee JY, Shim JI, Kim TH, Choi HS, Ahn HY, Kim KW, Park DJ, Park YJ, Yi KH. Prevalence of thyroid nodules and their associated clinical parameters: a large-scale, multicenter-based health checkup study. Korean J Intern Med. 2017 Jul 7.
- Anil C, Akkurt A, Ayturk S, Kut A, Gursoy A. Impaired glucose metabolism is a risk factor for increased thyroid volume and nodule prevalence in a mild-to-moderate iodine deficient area. Metabolism. 2013; 62(7):970-5.
- Caturegli P, Kimura H, Rocchi R, Rose NR. Autoimmune thyroid diseases. Curr Opin Rheumatol. 2007; 19:44–48.
- Lorini R, Gastaldi R, Traggiai C, Perucchin PP. Hashimoto's thyroiditis. Pediatr Endocrinol Rev 1. 2003; (Suppl 2):205–211.
- Krysiak R, Okopien B. Coexistence of primary aldosteronism and Hashimoto's thyroiditis. Rheumatol Int. 2012; 32(8):2561-3.
- Sabbadin C, Mian C, Nacamulli D, Donà G, Presotto F, Betterle C, Boscaro M, Bordin L, Armanini D.Association of primary aldosteronism with chronic thyroiditis. Endocrine. 2017; 55(1):303-306.