Information
Journal Policies
Effect of Hypertriglycridemia on the Frequency of Albuminuria in Saudi Populaion with Type 2 Diabetes Mellitus
Khalid S. Aljabri1*, Samia A. Bokhari1, Muneera A. Alshareef1, Patan M. Khan1, Hesham Abu Alsaoud1, Mohammad Jalal1, Rania Safwat1, Rehab Borae1, Bandari K. Aljabri2
2.College of medicine, Um Al Qura University, Makkah, Kingdom of Saudi Arabia.
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Diabetes is one of the most common chronic diseases. The development of albuminuria in type 2 diabetes (T2DM) might be induced by hypertriglycridemia.
The study was retrospective conducted at the Primary Health Care Clinics at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia. A total of 1590 Saudi with T2DM were randomly selected.
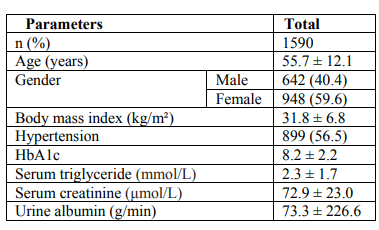
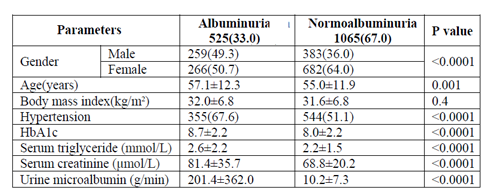
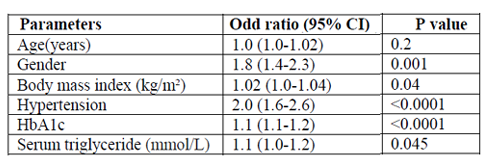
Total of 1590 patients with T2DM included in this study; 642 (40.4%) male and 948 (59.6%) female with mean age 55.7 ± 12.1 years. Hypertension was present in 899 (56.5%). Mean BMI 31.8 ± 6.8, HbA1c was 8.2± 2.2, mean TG was 2.3 ± 1.7 mmol/l and mean albumin excretion rate was 73.3 ± 226.6. Albuminuria was present in 525 (33.0%) and was significantly more prevalent in female (50.7%) with female predominance (sex ratio male: female) 1:1.1. Cases with albuminuria have significantly higher HbA1c compared to normalbuminuria, 8.7±2.2 vs. 8.0±2.2 respectively, p< 0.0001. HTN with albuminuria was more frequent in 355(67.6%) of albuminuria group with odd ratio 2.0 (1.6-2.6), p< 0.0001. The mean triglycerie is higher in age groups 30-39 and 40-49 years with significant male predominant across age groups.
We conclude that hypertriglyceridemia should be considered as a potential risk factor and as a diagnostic biomarker to be used in conjunction with other biochemical markers for early diagnosis, assessment, and follow-up of albuminuria.in diabetic patients by using a continuing, comprehensive and coordinated ap-proach.
Keywords: Type 2 diabetes, albuminuria and hypertriglycridemia
1. INTRODUCTION
Diabetes mellitus remains a challenge to public health worldwide. Diabetic nephropathy is the leading cause of end stage renal disease [1]. Moderately increased albuminuria formerly known as microalbuminuria is the first clinical sign of involvement of kidneys in patients with Type 2 diabetes (T2DM). It is also responsible for about third of all patients requiring renal replacement therapy. The development of diabetic nephropathy is characterized by progressive increase in the excretion of protein particularly albumin. Urinary albumin leakage is a manifestation of generalized vascular damage [2]. The estimation of the burden of albuminuria and its strength of association with cardiovascular disease would guide the management of public health programs for prevention and management of chronic disease.
Such targeted strategies could be particularly useful in population shown to be at high risk of cardiovascular disease, diabetes or micro albuminuria [2].
Diabetic dyslipidemia is common in T2DM and is characterized by high levels of fasting triglycerides (TG), low high density lipoprotein cholesterol levels, and predominance of small, dense low density lipoprotein cholesterol particles [3]. Moreover, the majority of patients with T2DM show high and prolonged postprandial lipemia after meals [4]. Hyperlipidemia has been found to be a risk factor for albuminuria in patients with diabetes [5]. Specifically, TG levels have been indicated as a strong independent determinant of micro albuminuria and macro albuminuria in diabetes and have been reported to be an important contributing factor in the progression of diabetic nephropathy [6,7]. Elevated triglyceride-rich lipoproteins, a key factor of altered lipid profile in diabetic nephropathy, is present even in the earlier stages of renal disease.
Although T2DM is more common in Saudi Arabia than in Europe, very little is known about complications and their risk factors in Saudi Arabia. There have been few studies on the influence of hypertriglyceridemia on albuminuria in Saudi populations. In this study we report on the frequency of albuminuria in patients with T2DM and hypertriglyceridemia attending a primary care clinics in Saudi Arabia.
2. METHODS
The study is a retrospective conducted at the Primary Health Care Clinics at King Fahad Armed Forces Hospital. A total of 1590 Saudi with T2DM were randomly selected. The demographic data and medical history were documented. Blood Pressure readings were within a gap of 15 minutes using a mercury sphygmomanometer by palpation and auscultation method in right arm in sitting position. Two readings, 15 min apart, were taken and the average of both the readings was taken for analysis. Hypertension (HTN) was also diagnosed based on anti HTN medications or having a prescription of antihypertensive drugs and were classified as Hypertensive irrespective of their current blood pressure reading or if the blood pressure was greater than 140/90 mmHg i.e. systolic BP more than 140 and diastolic BP more than 90 mm of Hg – Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines.8 HbA1c was expressed as percentage. High performance liquid chromatography was used. Albuminuria was assessed by measurement of mean albumin excretion rate (AER) on timed, overnight urine collections. We use a polyclonal radioimmunoassay for albumin measurement.
Albuminuria was defined as AER ≥30 g/min in overnight urine collections (equivalent to ≥30 mg/g creatinine in a random spot sample) [9]. The method used for determining TG levels in the laboratory was the Enzmatic method.
3. STATISTICAL ANALYSIS
Univariate analysis of baseline demography and clinical laboratory were accomplished using unpaired t-test. Chi square(X2) test were used for categorical data comparison. The independent relationship between risk factors and the odds ratio of having albuminuria were analyzed using logistic regression. All statistical analyses were performed using SPSS Version 22.0. All P values were based on two-sided tests. P < 0.05 was considered to be significant.
4. RESULTS
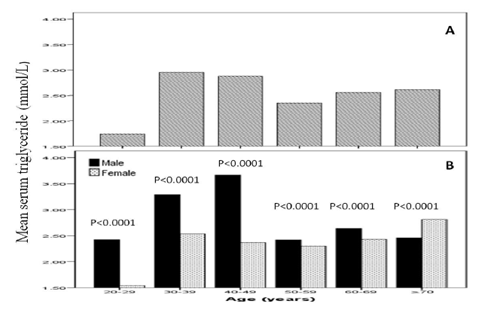
Total of 1590 patients with T2DM included in this study; 642 (40.4%) male and 948 (59.6%) female with mean age 55.7 ± 12.1 years, table 1. Hypertension was present in 899 (56.5%). Mean BMI 31.8 ± 6.8, HbA1c was 8.2± 2.2, mean TG was 2.3 ± 1.7mmol/l and mean albumin excretion rate was 73.3 ± 226.6. Albuminuria was present in 525 (33.0%) and was significantly more prevalent in female (50.7%) with female predominance (sex ratio male: female) 1:1.1, table 2. Cases with albuminuria have significantly higher HbA1c compared to normalbuminuria, 8.7±2.2 vs. 8.0±2.2 respectively, p< 0.0001. HTN with albuminuria was more frequent in 355(67.6%) of albuminuria group with odd ratio 2.0 (1.6-2.6), p < 0.0001, table 2 and 3. Figure for the mean TG according to age groups stratified by gender in patients with albuminuria showed the mean TG is higher in age group 30-39 and 40-49 years with significant male predominant across age groups.
5. DISCUSSION
Proteinuria is a well-known marker for renal disease [10]. The association of heavy proteinuria with edema, hypoalbuminemia, hyperlipidemia is also well recognized [11]. Over the past two decades, evidence for a direct adverse impact by proteinuria on the progression of chronic kidney disease to end-stage renal failure has accumulated [12]. However, these findings were obtained at stages with extensive renal injury and may have represented secondary effects.
Proteinuria is also associated with increased risk of atherosclerotic cardiovascular disease [13]. Hyperlipidemia is a metabolic abnormality frequently associated with diabetes mellitus. Its prevalence is variable, depending on the type and severity of diabetes, glycemic control, nutritional status, age and other factors. The most characteristic lipid abnormality in diabetics is hypertriglyceridemia [14].
This study based on the data collected from 1590 patients in the department of primary care at a tertiary care Hospital at Jeddah, Saudi Arabia showed significant increased albuminuria with 33% in Type 2 diabetic patients. Dyslipidemia is common among T2DM patients. Raised serum TG levels often precede the onset of T2DM for many years [15]. Hypertriglyceridemia and hyperglycemia are associated with an increased risk of future moderately increased albuminuria. Experimental and clinical studies have shown a strong association between hypertriglyceridemia and diabetic nephropathy [16]. Lipids may induce renal injury by stimulating Transforming growth factor-beta, thereby inducing the production of reactive oxygen species and causing damage to the glomeruli and glomerular glycocalyx [17]. In experimental animals fed a high fat diet which suggested that lipotoxicity might represent a major mechanism in renal disease by inducing glomerulosclerosis and tubulo-interstitial injury, associated with renal lipid accumulation [18] Insulin affects the liver Apo lipoprotein production. It regulates the enzymatic activity of lipoprotein lipase and Cholesterol ester transport protein. All these factors are likely cause of dyslipidaemia in Diabetes mellitus with albuminuria. Moreover, insulin deficiency reduces the activity of hepatic lipase and several steps in the production of biologically active lipoprotein lipase may be altered in Diabetes mellitus [19]. In our study, fasting serum TG were significantly associated with increased albuminuria as shown present in mean fasting serum TG level of 2.6±2.2 mmol/l and absent among those with 2.2±1.5 mmol/l, p< 0.0001. Increased TG have been associated with microalbuminuria, although associations with lipid abnormalities were found to be more marked in patients with macroalbuminuria [20]. With respect to the pediatric populations with diabetes, data from the Oxford Regional Prospective Study showed that the prevalence of microalbuminuria increased across tertiles of total cholesterol, and a German study has shown a predictive value of TG on the development of persistent microalbuminuria [21,22]. A time of exposure to elevated triglyceride levels >1.7 mmol/l (150mg/dL) predicts incident moderately increased albuminuria. In a study carried out by Gomes where total of fifty patients were enrolled. Moderately increased albuminuria was present in 10% of the patients. No difference concerning serum lipids were found in comparison between normalbuminuria and moderately increased albuminuria patients. A total of 4% of all patients had elevated triglyceride levels [23]. Another study showed out of total 184 cases, seventy six subjects, 41.3% had moderately increased albuminuria. There was no significant difference between subjects with and without moderately increased albuminuria with regards to triglyceride concentrations [24]. This was in contradiction to our study.
In our study more females 50.7% had MA as compared to males 49.3%.It was contradictory to study done by Hussain S that showed male dominance.
A metaanalysis of epidemiological data has shown that TG is an independent cardiovascular risk factors and evidence has accumulated over the past 10 years showing the importance of TG as an independent risk factor for coronary artery disease [25-27]. In Asian populations, nonfasting TG levels predict the incidence of coronary heart disease [28]. Also in Asian populations, TG appears to be more important than low density lipoprotein and high density lipoprotein as the major lipid parameters related to atherogenesis in the presence of hypertension [29]. TG levels increase with HTN [30]. This relationship persists in patients across patients with HTN, which indicates a biological interrelation between HTN and TG [31]. In our study, we identified a significant interaction between TG and HTN on albuminuria demonstrating a role for therapy targeting TG and HTN in early renal impairment in hypertensive diabetic patients.
A clinic based study introduces referral bias, is one of the limitations of this study. This could have introduced some degree of referral bias. However the prevalence of albuminuria is similar to that reported in other studies. A single urine spot collection with semi quantitative dipstick determinations was used to detect albuminuria. The American Diabetes Association guidelines stated that this technique has acceptable sensitivity and specificity, but recommend that several collections should be done in a 3 to 6 month period before diagnosing a patient as having albuminuria [32].
6. CONCLUSION
We conclude that hypertriglyceridemia should be considered as a potential risk factor and as a diagnostic biomarker to be used in conjunction with other biochemical markers for early diagnosis, assessment, and follow-up of albuminuria.
7. ACKNOWLEDGEMENT
The author would like to thank all colleagues from the department of primary care for helping in data collection.
References
- Cordonnier D, Bayle F,Benhamou P. Future trends of management of renal failure in diabetic. Kidney Int. 1993; 43:8-132.
- Deckert T, FeldtRasmussen B, Borch Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The steno hypothesis. Diabetologia 1989;32:219-26
- Betteridge, D. J. 1999. Diabetic dyslipidaemia. Eur. J. Clin. Invest.b29 (Suppl. 2): 12–16.
- Katsilambros, N. 1995. Postprandial triglyceridaemia. Diabet. Med. 12: 451–452.
- Hovind P, Tarnow L, Rossing P, Jensen BR, Graae M, Torp I, et al. Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: Inception cohort study. BMJ 2004; 328:1105.
- Kim DM, Ahn CW, Park JS, Cha BS, Lim SK, Kim KR, et al. An implication of hypertriglyceridemia in the progression of diabetic nephropathy in metabolically obese, normal weight patients with type 2 diabetes mellitus in Korea. Diabetes Res Clin Pract 2004; 66 Suppl 1:S169-72.
- Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR; UKPDS Study Group, et al. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective diabetes study 74. Diabetes 2006; 55:1832-9.
- ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/A SH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;Nov 13
- National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease. Part 5: Evaluation of laboratory measurements for clinical assessment of kidney disease. Am J Kidney Dis.2002;39: S76-S92
- Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis 1999; 33:1004-1010.
- Orth SR, Ritz E. The nephrotie syndrome. N EngI J Med 1998; 338: 1202-1211.
- Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N EnglJ Med 1998; 339:I448-1456.
- Yuyun MF, Khaw K-T, Luben R, et al. A prospective study of microalbuminuria and incident coronary heart disease and its prognostie significance in a British population—the EPIC-Norfolk Study. Am J Epidemiol 2004; 159:284-293.
- Rathore, R., Rathore, S., Sharma, N., et al. Effect of hyperglycemia on Postprandial lipid profile in Type 2 Diabetes. (2010) Pharmacology online 3: 291-297.
- Fruhat JC, Sacks F, Hemman MP et al: The residual risk reduction initiation: a call to action to reduce vascular risk in patients with dyslipidemia. Am J Cardiol vol. 102; 2008:341-445.
- Bardini G, Inmocenti M, Rutella E.Variability of triglyceride level and incidence of microalbuminuria in Type 2 Diabetes. J Clin Lipidol 2016 Jan-Feb, 10(11):109-115
- Rutledge JC, Ng KF, Aung HH, Wilson DW. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nature Reviews Nephrology, 2010; 6: 361-370.
- Weinberg JM. Lipotoxicity. Kidney Int. 2006; 70: 1560–1566.
- Ram Vinod Mahatol, Prajwal Gyawali, Pramod P Raut, Prashant Regmi, Khelanand P. Singh, Dipendra R Pandeya et al. Association between glycaemic control and serum lipid profile in type 2 diabetic patients: Glycated haemoglobin as a dual biomarker. Biomedical Research, 2011; 22 (3): 375-380.
- Mattock MB, Cronin N, Cavallo-Perin P. Plasma lipids and urinary albumin excretion rate in type 1 diabetes mellitus: the EURODIAB IDDM Complications Study. Diabet Med 2001; 18:59–67.
- Abraha A, Schultz C, Konopelska-Bahu T. Glycaemic control and familial factors determine hyperlipidaemia in early childhood diabetes: Oxford Regional Prospective Study of Childhood Diabetes. Diabet Med 1999; 16:598–604.
- Raile K, Galler A, Hofer S. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 2007; 30:2523–8.
- Gomes MB, Luchetti MR, Gazolla H, Dmaetz J, Lobao V, Stum J. Lipids, microalbuminuria and systemic blood pressure in patients with insulin dependent diabetes mellitus. Arq Bras Cardiol; 1997 Feb; 68(2):85-9
- Alizaid AA, Sobki S, Desilva V. Prevalance of micoalbuminuria in Saudi Arabia with Non-Insulin dependent diabetes mellitus;: a clinic based study. Diabetes Res Clin Pract 1994 Dec 16; 26(2):115-20
- Ahmad I, Miller M. Triglycerides and coronary heart disease: A global perspective. J Cardiovasc Risk 2000; 7:303-7.
- Gaziano JM, Hennekens CH, O’Donnell CJ, Breslow JL, Buring JE. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation 1997; 96:2520-5.
- Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Relation of high TG-low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease. An 8-year follow-up in the Copenhagen male study. Arterioscler Thromb Vasc Biol 1997; 17:1114-20.
- Iso H, Naito Y, Sato S, Kitamura A, Okamura T, Sankai T, et al. Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am J Epidemiol 2001; 153:490-9.
- Yamamoto A, Temba H, Horibe H, Mabuchi H, Saito Y, Matsuzawa Y, et al. Life style and cardiovascular risk factors in the Japanese population – From an epidemiological survey on serum lipid levels in Japan 1990 part 2: Association of lipid parameters with hypertension. J Atheroscler Thromb 2003; 10:176-85.
- Bønaa KH, Thelle DS. Association between blood pressure and serum lipids in a population. The Tromsø study. Circulation 1991; 83:1305-14.
- Saidu H, Karaye KM, Okeahialam BN. Plasma lipid profile in Nigerians with high – Normal blood pressure. BMC Res Notes 2014; 7:930.
- American Diabetes Association. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes. Diabetes Care. 2018 Jan; 41(Supplement 1): S105-S118.