Information
Journal Policies
Latest Therapeutics in Plaque Psoriasis: A Review
Omeed M. Memar1*, Benjamin Caughlin2
2.Department of Surgery / Division of Otolaryngology, John H. Stroger, Jr. Hospital of Cook County, Division of Facial Plastic and Reconstructive Surgery, University of Illinois Health Hospital System, Chicago, IL. Kovak Cosmetic Center, Oakbrook Terrace, Illinois.
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Psoriasis is a common systemic inflammatory syndrome that affects numerous organs. It is most evident on the skin, and hence garners the greatest attention. Historically, the treatments for psoriasis made incremental advancements. Since the immunology of the disease has been better understood and the advent of therapeutic monoclonal antibodies, a flood gate of new therapies has been opened. In this review, we discuss the immunology of psoriasis and the recombinant therapies along with the newest therapies on the horizon.
Keywords: Psoriasis, certolizumab, secukinumab, ixekizumab, brodalumab, guselkumab, tildrakizumab, risankizumab, mirikizumab, tofacitinib.
1. Introduction
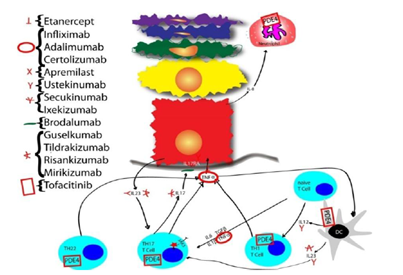
Psoriasis is an inflammatory dysregulation that affects about 2% of the population of the United States, and has a strong genetic and environmental pathogenesis. It affects multiple organs systems, most visible being the skin [1]. The Skin Associated Lymphoid Tissue (SALT)[2] is a complex system to protect the skin by producing inflammation. When this system breaks down and becomes dysregulated, inflammation can cause damage. Psoriasis has a strong immunogenetic component. Psoriasis can present in half of siblings when both parents have psoriasis; in 16% of siblings when one parent has psoriasis; and 8% of siblings when neither parent has psoriasis [3]. Amongst twins with psoriasis, identical twins have a 70% concordance rate and fraternal twins have a 20% concordance rate [4]. The genes most associated with psoriasis are HLA-Cw65, HCR*WWCC [6] HLA-associated S gene [7]: these three loci are collectively referred to as the psoriasis susceptibility 1 locus (PSORS1), and reside on 50% of psoriasis cases. The initiating factor that leads to the dysregulation of SALT is in psoriasis has not been identified. However, a bacterial hypothesis has been proposed, and bacterial infections have been documented to initiate psoriasis [8]. In the initial phase, antigen presenting cells (APCs), in the presence of interferon γ (IFNγ), IL-6 and IL1β, migrate to regional lymph nodes, and reside there. In the lymph node, an antigen is presented to a naïve T Cell in the presence of IL12, IL23, and TNFα. This converts the naïve T Cell into either a TH22, TH1, or TH17 cell. (Figure 1) These T cells migrate back to the skin and reside in the dermis, releasing different cytokines. These cytokines have been a great target of biologics, and hence therapy. The most commonly used biologics target TNFα, and have been very effective. However, one of the most important pathways in the pathogenesis of psoriasis is IL17, and hence a great deal of attention is paid to IL17. In this review, the newest and next generation medications will be discussed in the therapeutics of psoriasis.
TNFα is found in high concentrations in psoriatic plaques and patient serum. TNFα increases angiogenesis and blood vessel permeability of the skin, dendritic cell maturation, and lymphocyte migration to the regional lymphnodes[9].The micaceous scales of psoriasis are made under the influence of TNFα induction of keratinocyte proliferation and increase in protease inhibitors [10]. Therefore, the neutralization of TNFα reverses both the inflammatory and hyperkeratotic aspects of psoriasis.
Background: Etanercept was approved by the FDA in 2004 for plaque psoriasis. It is a 150 kDa recombinant fusion protein of human soluble TNF receptor 2 and the Fc portion of human IgG1, as a monoclonal antibody [11]. In 2008, the drug got a black box warning, due to different serious infections.
Mechanism of Action: The mechanism of action is binding soluble TNFα and acting as a decoy sponge, adsorbing the soluble TNFα and preventing them from binding functional receptors. It is given as a subcutaneous injection.
Dosing: Starting dosage is 50mg twice daily for 3 months and maintenance dose is 50mg weekly thereafter. In pediatric patients 4-17, the doe is weight based, with patients 138 lbs and below 50mg weekly and those below 138 lbs, .8mg/kg weekly. Patients need to be evaluated for Tb prior to initiation of therapy.
Efficacy: In the United states, the patent has been extended, but in Europe that is not the case. In 2015, Samsung and Biogen introduced a biosimilar version of Etanercept and named it Benapali and Sandon and Novartis introduced another biosimilar version called Erelzi. Pregnancy category B. A 5-year pediatric and adolescent study revealed that the most common adverse effects were upper respiratory tract infections (37%), nasopharyngitis (26%) and headaches (21.5%). Risk of melanoma and non-melanoma skin cancer risk is elevated [12]. Following patients till week 264, PASI 75 was 60-70% and PASI 90 was 30-40% [13].
Background: Infliximab was approved by the FDA in 2006 for psoriasis. It is a 149kDa recombinant fusion protein of mouse Fab and Fc portion of human IgG1 as a monoclonal antibody.
Mechanism of Action: The target is TNFα. It is an intravenous infusion that scavenges TNFα.
Dosage: In the induction phase, it is given at 5mg/kg on weeks 0, 2 and 6, and then maintained at 5mg/kg every 8 weeks. Patients need to be evaluated for Tb prior to initiation of therapy.
Efficacy: The largest phase III trial showed that76% of patients on infliximab achieved PASI 75, compared to only 2% of the placebo.14Others corroborated the PASI 75 score at 10 weeks: 79%15 and 77%.16Pregnancy Category B. Risk of melanoma and non-melanoma skin cancer risk is elevated [4].
Background: Adalimumab is a fully humanized monoclonal antibody FDA approved for psoriasis in 2008.
Mechanism of Action: Fully humanized anti-TNFα binds and neutralizes active TNFα.
Dosing: Adalimumab starter dose is 80mg day 1, 40mg day 8, 40mg day 22, then maintained on 40mg every other week. Patients need to be evaluated for Tb prior to initiation of therapy. Pregnancy Category B.
Efficacy: In adults who were unresponsive to other systemic therapies, after 12 weeks of adalimumab, 87% achieved PASI 75 [17]. In a larger 16 week study, patients who were non-responders to etanercept achieved PASI 75 in 53.8-65.7% of patients [18]. In children and adolescents, 0.8mg/kg dosing of adalimumab every 2 weeks was more efficacious than .1-.4mg/kg methotrexate, yielding a PASI 75 of 58% and 32%, respectively at 16 weeks [19].
Background: Certolizumab is a chimeric monoclonal human Fab against TNFα and polyetheleneglycol (PEG). It is the only anti-TNF antibody for psoriasis missing an Fc portion [20].
Mechanism of Action: It is FDA approved for moderate-severe plaque psoriatic in 2018.
Dosing: The dose is 400mg subcutaneous injections every two weeks for plaque psoriasis. Patients need to be evaluated for Tb prior to initiation of therapy.
Efficacy: PASI 90 was achieved at 12 weeks with the 400mg dosage in 34% while only 27.1% of those on etanercept achieved PASI 90. PASI 75 was maintained at 400mg every 2 weeks for 87.1%. The most common side effects were nasopharyngitis [21].Pregnancy Category B. The Fc portion of antibodies are responsible for mother to infant transmission of antibodies. Since certolizumab lacks an Fc portion, that might explain the low transmission of this drug to infants in utero and through breast milk [22].
Psoriasis is known to be part of a metabolic syndrome. Metabolic syndrome is associated with higher cardiovascular incidents. Patients with psoriasis have been shown to have higher cardiovascular risks [23]. A comparison of adults receiving TNF inhibitors versus methotrexate for psoriasis showed a cardiovascular risk of 1.45% for the TNF inhibitor group and 4.09% for the methotrexate group. This indicates that TNF inhibition reduces cardiovascular incidents [24].
Background: Apremilast was FDA approved for moderate to severe psoriasis in 2014.
Mechanism of Action: It is a small molecule that blocks the signal transduction of phosphodiesterase type 4, thereby increasing the intracellular cAMP levels in inflammatory cells. The elevated cAMP results in reduced production TNFα, IL23, interferon (IFN) γ and an elevation of IL10[25].
Dosing: There is no need for Tb testing. The dosage begins with 10mg QD day1, 10mg BID day 2, 10mg in AM and 20mg in PM day 3, 20mg BID day 4, 20mg in AM and 30mg in PM, and 30mg BID thereafter. The most common side effect is GI problems; the majority subside after two weeks.
Efficacy: After 16 weeks of therapy, PASI 75 was achieved by 33% of apremilast-treated patients versus 5% of the placebo group [26]. There is a slight increase of depression (.8% vs .4 in placebo), and 12% of patients lost 5-10% weight, compared to 5% of placebo-treated patients having any weight loss. Furthermore, apremilast is metabolized by CYP 450, and inducers, like rifampin or phenytoin can reduce drug levels [27]. Pregnancy category C.
Background: IL12 is a heterodimer, and there are two separate genes. The one that is hinged by p40 is involved in psoriasis. IL12 is made by Dendritic Cells (DC) amongst other cells, and facilitate a TH1 profile. Monoclonal antibody formation to the p40 subunit that binds IL12/IL23 shows therapeutic efficacy in psoriasis. There are two such compounds, first is Ustekinumab, and the second is Briakinumab. Briakinumab was discontinued in 2011 for approval for psoriasis. Ustekinumab is a humanized IgG1 monoclonal antibody of 148.6kDa, and is expressed in aSp2/0 murine myeloma cell line. It was FDA approved for moderate-to-severe psoriasis in 2009.
Mechanism of Action: It has high affinity and specificity to the 40kDa p40 subunit of IL12 and IL23 heterodimeric proteins.
Dosing: It is a subcutaneous injection dosed by body weight. Patients weighing 100kg or less get 45mg doses, and those weighing greater than 100kg get 90mg doses. The intervals of injections area at weeks 0, 4, and 12. The maintenance injections are administered every 12 weeks. Patients need to be evaluated for Tb prior to initiation of therapy.
Efficacy: In a head to head study, ustekinumab was more efficacious than etanercept. At the twelfth week, 73.8% of 90mg ustekinumab vs56.8% of etanercept 50mg twice weekly achieved PASI 75.28 The most common adverse reaction of ustekinumab was nasopharyngitis (7-8%) [29]. Five year follow up on 12,000 patients did not show an increase in life-threatening infections, cancer or mortality rate [30]. This was corroborated that ustekinumab does not increase risk of malignancy in a 5-year study [31]. Pregnancy Category B.
IL23 is an important inducer of IL17 upregulation, and therefore it plays a key role in the pathogenesis of psoriasis.
Background: Guselkumab is a monoclonal antibody directed to IL23 p19. It was FDA approved for moderate to severe plaque psoriasis inn 2017.
Mechanism of Action: Guselkumab targets the p19 protein, and is more specific for the Th17 cells, as opposed to the Th1 cells, which are important in certain infection control [32].
Dosing: Guselkumab is started at 100mg subcutaneous injections at week 0 and 4, then once every 8 weeks. Patients need to be evaluated for Tb prior to initiation of therapy.
Efficacy: At 16 weeks, PASI 90 was achieved by73.3% onguselkumab, while only 49.7% on adalimumab [33]. Furthermore, 66.1% of those not responding to adalimumab, when switched to guselkumab, achieved PASI 90 at 48 weeks [34].Pregnancy category not assigned.
Background: Tildrakizumab was approved for adults with moderate to severe plaque psoriasis by the FDA in 2018. Patients need to be evaluated for Tb prior to initiation of therapy.
Mechanism of Action: Tildrakizumab is a monoclonal antibody directed to IL23 p19
Dosing: A subcutaneous injection of 100mg at week 0, 4 and every 12 weeks thereafter. Patients need to be evaluated for Tb prior to initiation of therapy.
Efficacy: PASI 90 was seen in 37% at week 12, and 54% at week 28 [35]. The efficacy is not fully realized till week 28. Pregnancy category not assigned. PASI 100 was seen in 12-14% [47].
Risankizumab is a monoclonal antibody directed to IL23 p19 in phase III clinical trials, and not yet FDA approved for psoriasis. It is one of the most promising of psoriasis treatments to come. Its dosing will be week 0, week 4 and every four months from then on. A study with ustekinumab showed that at 12 weeks, 78.6% of those receiving risankizumab attained PASI 90, while only 40% of ustekinumab treated group achieved PASI 90 [36].
Mirikizumab is a humanized monoclonal IgG4κ against IL23. It is in clinical trials and not yet approved for psoriasis. Its dosing regimen is more often than the other IL23 inhibitors. Results are pending.
L17 is a family of molecules, including IL17A, IL17B, IL17C, IL17D, IL17E (IL25) and IL17F. IL17 A and F have the highest homology.IL17 is an important pro-inflammatory cytokine released by T Cells, especially in response to IL23. IL17 is upregulated in psoriasis [37].
Background: Secukinumab is a recombinant human monoclonal IgG1/κ antibody, weighing 151kDa. It is expressed in Chinese Hamster Ovary (CHO) cell system.
Mechanism of Action: Secukinumab binds IL17A and neutralizes the cytokine.
Dosing: Secukinumab is a subcutaneous injection administered by weight. For those less than 60 kg, the dose is 150mg and those weighing greater than 60kg, the dose is 300mg on weeks 0, 1, 2, 3, and 4. The maintenance dose is 300mg every 4 weeks [38].Patients need to be evaluated for Tb prior to initiation of therapy.
Efficacy: The 300mg dose is superior to the 150mg dose, and both doses are more efficacious than etanercept [22] and ustekinumab [39]. At week 12, 300mg secukinumab dosage gave PASI 75 to 81.6% of patients, and at week 52, 74.3% were at PASI 75.40 In a head to head study with ustekinumab, 300mg secukinumab had PASI 74 at 4 weeks for 50% of patients, while ustekinumab showed it in 20.6%. Furthermore, at 16 weeks, secukinumab achieved PASI 90 in 76% of patients, while 67.7% of ustekinumab patients achieved PASI 90. Interestingly, PASI 100 was achieved in 46% of secukinumab and 36% of ustekinumab [41].
The most common adverse event of secukinumab is nasopharyngitis (11.4-12.3%) and mucocutaneous candidiasis. It is not efficacious for inflammatory bowel disease, and can exacerbate Crohn’s disease [42]. There is also a higher rate of mucocutaneous candidiasis. Pregnancy Category B.
Background: Ixekizumab is a 146kDa monoclonal antibody to IL17A.
Mechanism of Action: It binds and neutralizes IL17A
Dosing: Patients need to be screened for Tb prior to starting. The initial loading dose is 160mg, and then 80mg every 2 weeks for the first 12 weeks. Then the dose is switched to 80mg every 4 weeks. Patients need to be evaluated for Tb prior to initiation of therapy.
Efficacy: At the above dosage, after 12 weeks, 89.7% ixekizumab patients achieved PASI 75, while only 41.6% of etanercept 50 mg twice weekly [43]. However, at 60 weeks of treatment, 73.8% of ixekizumab maintained PASI 75 [44]. Rate of mucocutaneous candidiasis was 3.4%, inflammatory bowel disease was .3%, and grade ¾ neutropenia was 3% [26]. Pregnancy status is pending. An extensive study of IL17 antagonists’ role in inflammatory bowel disease (IBD) was undertaken, and concluded that IBD is rare in IL17 antagonist treated patients [45].
Background: ALX-0761/M1095 is a nanobody targeting IL17A/F and bimekizumab is a humanized monoclonal IgG1 antibody targeting IL17A/F. Both are not yet FDA approved. Only initial studies have been completed, which showed 56% PASI 100 in a small study [46].
Background: Brodalumab is a monoclonal IgG2κ antibody.
Mechanism of Action: Brodalumab is an inhibitory antibody, binding the IL17 receptor A (IL17RA). This blocks IL17A, IL17F, IL17A/F, IL17C and IL17E
Dosing: It is a subcutaneous injection of 210mg administered on weeks 0, 1, 2, and then Q 2 weeks. Patients need to be evaluated for Tb prior to initiation of therapy.
Efficacy: Due to a risk of suicide, a Risk Evaluation and Mitigation Strategy (REMS) program is required, in which prescribers and pharmacies are registered with the program and the patient needs to consent to taking the medicine. At 12 weeks, 86% of patients on brodalumab achieved PASI 75. Forty four percent achieved PASI 100, while only 22% of those on ustekinumab [47]. At the highest doses, PASI 100 was achieved in 60% [48].Crohn’s disease may be aggravated by brodalumab, and those patients would avoid this drug. Adverse effects included mucocutaneous candidiasis, serious infections and depression were similar to that of ustekinumab, while Crohn’s was .1% vs 0 in ustekinumab. Four deaths occurred on brodalumab, one stroke, and two cardiac arrest and one in a motor vehicle accident [29]. Pregnancy category not assigned.
Tofacitinib is a JAK1 and JAK 3 inhibitor. The JAK/STAT pathway takes extracellular information and transmits it to the nucleus. Tofacitinib blocks this process. It is FDA approved for psoriatic arthritis and in phase II trials for plaque psoriasis. A 16 week study of 29 plaque psoriasis patients, 75% taking tofacitinib 10mg BID achieved PASI 75, while 0% of the placebo did [49]. Pregnancy status is C. Patients need to be evaluated for Tb prior to initiation of therapy.
2. Conclusion
The immunopathology of psoriasis has given specific targets for therapeutics. The first biologic in the U.S was against CD2, which is present on activated T cells, however, the low efficacy of the anti-CD-2 therapy opened the way to the anti-TNF monoclonal antibodies. The anti-TNFs made PASI 75 an easily achievable goal. However, they came with certain side effects of immunosuppression. As the IL17 pathway and the cytokines involved in the secretion of IL17 became better elucidated, the anti-IL12/23 injectable proved more efficacious, while have less side effects. This has blossomed into direct medications that either block IL17 from contacting its receptor or all together neutralizing IL17A. The near future promises known intracellular compounds like the JAK inhibitors, but in the horizon are potential blockers of other cytokines that play a role in the pathogenesis of psoriasis, namely IL3650, and improvements in reaching current targets, i.e., IL17 or IL23 with reduced frequency of dosing and greater binding ability.
References
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013; 133(2):377-385.
- Memar OM, Arany I, Tyring S. Skin-Associated Lymphoid Tissue in Human Immunodeficiency Virus-1, Human Papillomavirus, and Herpes Simplex Virus Infections. Journal of Investigative Dermatology. 1995; 105:99S-104S.
- Watson W, Cann HM, Farber EM, Nall ML. The genetics of psoriasis. Arch Dermatol. 1972;105:197-207
- Brandrup F, Holm N, Grunnet N, Henningsen K, Hansen HE. Psoriasis in monozygotic twins: variations in expression in individuals with identical genetic constitution. Acta DermVenereol. 1982; 62:229-236.
- Mallon E, Bunce M, Wojnarowska F, Welsh K. HLA-CW*0602 is a susceptibility factor in type I psoriasis, and evidence Ala-73 is increased in male type I psoriatics. J Invest Dermatol.1997;109:183-186
- Asumalahti K, Veal C, Laitinen T, et al. Coding haplotype analysis supports HCR as the putative susceptibility gene for psoriasis at the MHC PSORS1 locus. Hum Mol Genet 2002;11:589-597
- Allen MH, Veal C, Faassen A, et al. A non-HLA gene within the MHC in psoriasis. Lancet 1999;353:1589-1590
- Gudmundsdottir AS, Sigmundsdottir H, Sigurgeirsson B, Good MF, Valdimarsson H, Jonsdottir I. Is an epitope on keratin 17 a major target for autoreactive T lymphocytes in psoriasis? Clin Exp Immunol 1999;117:580-586
- Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem. 2004; 279(31):32633-42.
- Gottlieb AB. Infliximab for psoriasis. J Am Acad Dermatol. 2003; 49:112-7.
- Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. The Journal of Experimental Medicine. 1991; 174 (6): 1483-89.
- Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. The Journal of Experimental Medicine. 1991; 174 (6): 1483-89.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3
- Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56:31.e1-.e15
- Cassano N, Loconsole F, Amoruso A, et al. Infliximab monotherapy for refractory psoriasis: preliminary results. Int J Immunopathol Pharmacol. 2004; 17:373-80.
- Smith CH, Jackson K, Bashir SJ, et al. Infliximab for severe, treatment-resistant psoriasis: a prospective, open-label study. Br J Dermatol. 2006; 155:160-9.
- Bissonnette R, Bolduc C, Poulin Y, Guenther L, Lynde CW, Maari C. Efficacy and safety of adalimumab in patients with plaque psoriasis who have shown an unsatisfactory response to etanercept. J Am Acad Dermatol. 2010;63(2): 228-234. doi: 10.1016/j.jaad. 2009.08.040.
- Ortonne JP, Chimenti S, Reich K, Gniadecki R, Sprogel P, Unnebrink K, et al. Efficacy and safety of adalimumab in patients with psoriasis previously treated with anti-tumour necrosis factor agents: subanalysis of BELIEVE. J Eur Acad Dermatol Venereol. 2011; 25(9):1012- 1020.
- Papp K, Thaçi D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017; 390:40-49.
- Baker T, Kevorkian L, Nesbitt A. Investigation into the binding affinity of certolizumab pegol to FcRn and functional consequences for FcRn-mediated transcytosis: comparison to infliximab, adalimumab and etanercept Ann Rheum Dis, 2013;72:A426.421-A42.
- Gottlieb AB, Blauvelt A, Thaçi D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from two phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J Am Acad Dermatol. 2018; 79:302-314.e6.
- Clowse ME, Förger F, Hwang C, et al. Minimal to no transfer of certolizumab pegol into breast milk: results from CRADLE, a prospective, postmarketing, multicentre, pharmacokinetic study. Ann Rheum Dis. 2017; 76:1890-1896.
- Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006; 298:321-328.
- Wu JJ, Guérin A, Sundaram M, Dea K, Cloutier M, Mulani P. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate. J Am Acad Dermatol. 2017; 76:81-90.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012; 83(12):1583-1590.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37-49
- Otezla (apremilast) prescribing information. Summit, New Jersey: Celgene Corp.; Dec, 2014.
- Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med 2010; 362: 118-128.
- Janssen Biotech, Inc. Stelara (ustekinumab) injection, for subcutaneous or intravenous use [prescribing information], https://www.Stelarai nfo.com/sites/www.stelarainfo.com/files/Prescr ibing_Information.pdf (2016, accessed 26 September 2016).
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol 2014; 13: 1441-1448
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017; 77:845-854.e5.
- Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol2007; 8: 950-957.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol 2017; 76: 405-417
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76: 418-431.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017; 390: 276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med 2017; 376: 1551-1560
- Van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009; 182:5836-45.
- Van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009; 182:5836-45.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol 2017; 76: 60.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med 2014; 371: 326.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol 2017; 76: 60
- Hueber W, Sands BE, Lewitzky S, et al.Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012; 61: 1693-1700.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015; 386: 541-551.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 2016; 375: 345-356.
- Reich K, Leonardi C, Langley RG, et al. Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: a presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials. J Am Acad Dermatol. 2017; 76(3):441-448.e442.
- Svecova D, Grenningloh R, Krueger J, et al. Safety and efficacy of multiple ascending doses of subcutaneous M1095, an anti-interleukin-17A/F bispecific nanobody, in patients with moderate-to-severe psoriasis: 5511. J Am Acad Dermatol. 2017; 76:AB224.
- Svecova D, Grenningloh R, Krueger J, et al. Safety and efficacy of multiple ascending doses of subcutaneous M1095, an anti-interleukin-17A/F bispecific nanobody, in patients with moderate-to-severe psoriasis: 5511. J Am Acad Dermatol. 2017; 76:AB224.
- Svecova D, Grenningloh R, Krueger J, et al. Safety and efficacy of multiple ascending doses of subcutaneous M1095, an anti-interleukin-17A/F bispecific nanobody, in patients with moderate-to-severe psoriasis: 5511. J Am Acad Dermatol. 2017; 76:AB224.
- Abe M, Nishigori C, Torii H, Ihn H, Ito K, Nagaoka M, Isogawa N, Kawaguchi I, Tomochika Y, Kobayashi M, Tallman AM, Papp KA. Tofacitinib for the treatment of moderate to severe chronic plaque psoriasis in Japanese patients: Subgroup analyses from a randomized, placebo-controlled phase 3 trial. J Dermatol. 2017; 44:1228-1237.
- Swindell WR, Beamer MA, Sarkar MK, Loftus S, Fullmer J, Xing X, Ward NL, Tsoi LC, Kahlenberg MJ, Liang Y, Gudjonsson JE. RNA-Seq Analysis of IL-1B and IL-36 Responses in Epidermal Keratinocytes Identifies a Shared MyD88-Dependent Gene Signature. Front Immunol. 2018; 9:80.