Information
Journal Policies
Contamination of Glass-Ionomer Cement with Bacterial Aerobes in Dental Setups: A Microbiological Study
Dr. Abdul Salam TA1, Dr. Rekha Prashanth Shenoy2, Dr. Ganesh Shenoy Panchmal2, Dr. Vinej Somaraj3*
2.Professor, Department of Public Health Dentistry, Yenepoya Dental College, Karnataka, India
3.Senior Lecturer, Department of Public Health Dentistry, Rajas Dental College & Hospital, Tamil Nadu, India
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Objectives: To analyse aerobic bacterial contamination of Glass-ionomer cement (GIC) during usage at clinical setups.
Materials and Methods: An in-vitro study was conducted to analyse aerobic bacterial contamination of GIC at five different dental setups (Three samples from each site). Three samples of sealed GIC served as controls. The samples were collected and subjected to microbiological tests and data obtained was subjected to statistical analysis. Descriptive statistics and Binomial test were used.
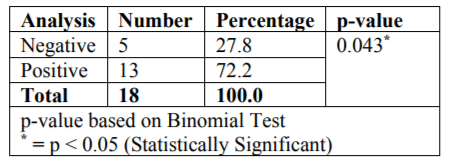
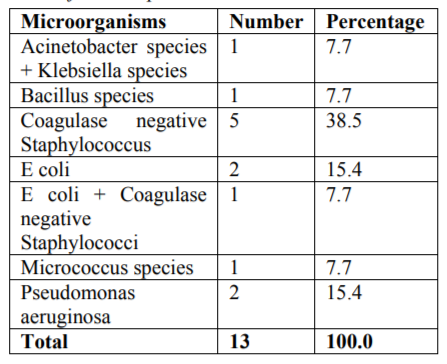
Results: Among the 18 samples analysed, 15 samples serving as cases showed positive results (Acineobacter species, Klebsiella species, Bacillus species, Coagulase-negativeStaphylococcus, E Coli, Micrococcus species and Pseudomonas Aeruginosa) with greatest contamination in Mobile Dental van (p =0.043).
Conclusion: The increasing GIC contamination in dental setups is mainly due to poor infection control methods, particularly during its manipulation on a mixing pad. Reviewing infection control guidelines and introducing dental mixing sheet dispensers could go a long way in helping to reduce the issue of contamination.
Keywords: Aerobic Bacteria, Contamination, Dental Setup, Glass-Ionomer Cement, Infection,Dental Science
1. Introduction
The dental clinic and hospital is an environment where disease transmission can occur easily. The dental practice is crucially impacted by cross-contamination that may principally be attributed to a wide range of microorganisms. This follows the standard pattern of transmission from patients to dentists and vice versa, which forms the basis for the council that practising dental professionals adhere to standard infection control protocol in order to prevent this. Dental professionals are at risk of infections caused by various microorganisms such as Staphylococci, Streptococci, Mycobacterium Tuberculosis, Human Immunodeficiency Virus (HIV), Hepatitis B Virus (HBV), Hepatitis C Virus (HCV),Herpes Simplex Virus type I (HSV I)[1].
The risk of HIV infection after the percutaneous exposure to HIV-infected blood was estimated to be 0.3%[2,3]. Similarly the prevalence of HBV infections ranges from 2% in Europe and USA to 8% in Asia[4] and that of HCV infection ranges from 0.1 – 1% in Northern Europe, 0.2 – 1.2% in Central Europe and 2.5 – 3.5% in Southern Europe[5]. Infections may be transmitted in the dental operatory through several routes, including direct contact with blood, oral fluids, or other secretions and indirect contact with contaminated instruments, operatory equipment and dental materials[6,7]. In the past, the cause of the contamination reported in regard to operating instruments and dental materials, was attributed to an array of microorganisms ranging from Staphylococcal species to Candida species[8–11]. Glass-ionomer cement (GIC) is one such dental restorative material used for dental filling and luting cement, and are frequently used due to its versatile properties and relative easiness[12,13]. GIC may get contaminated easily during clinical usage and even before usage with reported bacterial contamination of 20-30% in factory sealed GIC containers[14]. Despite reports of contamination in factory sealed packs of GIC, no studies have been conducted to shed light on potential higher microbial contamination in packs which are in active clinical use and this study aims at filling this void. Surprisingly, there is hardly any information available on such contamination and this study was carried out to analyse and compare aerobic bacterial contamination of GIC at different dental setups during its clinical usage.
2. Materials And Methods
After obtaining ethical clearance from the University Ethics Committee and permission from concerned dental setups, an in-vitro study was conducted to analyse the bacterial contamination of GIC during its clinical usage. Based on convenience sampling, one sample of all clinically used Glass-ionomer cements types (Type I: Luting cement, Type II: Restorative Cement and Type III: Liners and Bases) of same brand available in the Department of Public Health Dentistry (Sample B), Satellite centre-1 (Sample C), Satellite centre-2 (Sample D), Satellite centre-3 (Sample E) and Mobile dental van (Sample F) was collected (n=15) which served as cases. Three samples of sealed Glass-ionomer cement types served as control (Sample A), constituting a total sample of 18. All samples were collected aseptically and GIC with exceeded expiry dates were excluded. The three satellite centres chosen for the present study were functioning under the Department of Public Health Dentistry to maintain uniformity for the collection of samples.
One gram of each GIC type was collected in closed containers from different dental setups which were transferred and separated aseptically into 5ml sterile brain heart infusion broth in a biological safety cabinet. Each sample was then cultured, and incubated at 37°C for 48 hours and observed for any turbidity. When turbidity was noted one loop full of the culture was taken and a smear was prepared and stained by Grams staining method. Tubes which did not show any signs of growth were further incubated for 4 more days. When the grams stained smear showed the presence of a microorganism, a semi-quantitative culture is performed to quantify the number of bacteria in the sample.
Semi-quantitative culture: From the tubes that showed visible growth of a microorganism, as well as positive grams, stain findings, one loop (2mm wire loop, which can carry 0.01ml of broth) full of broth, was sub-culture donto brain heart infusion agar and was incubated for 24 – 48 hours at 37°C. The colonies formed on the agar surface were manually counted and multiplied by 100 to get a final concentration of organism in 1ml of the broth. The organisms were identified by standard procedures for the identification of the bacteria.
Data obtained were analysed using the Statistical Package for Social Sciences (SPSS) Version 21.0 software. Descriptive statistics and Binomial test were used. The significance level was set at 5%.
3. Results
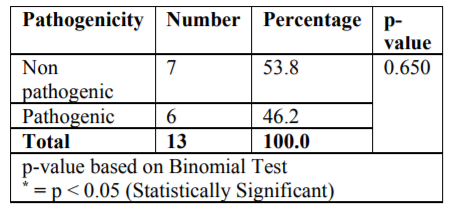
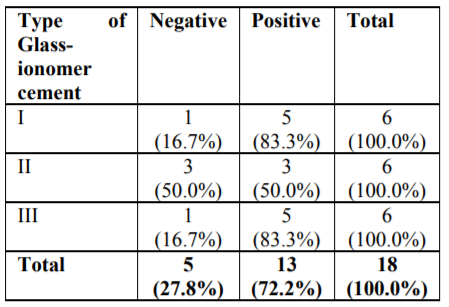
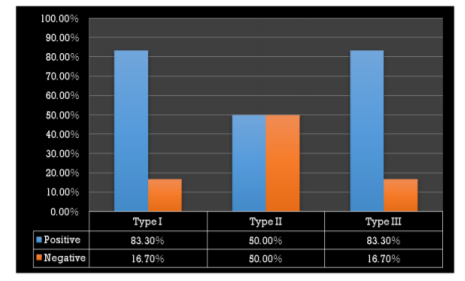
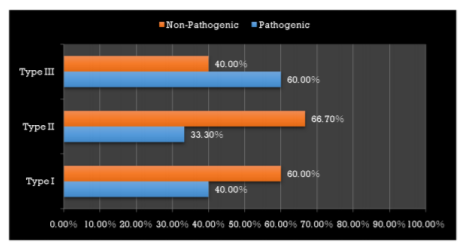
The microbiological study revealed that out of 18 samples analysed 13 samples showed bacterial contamination (p =0.043) which was statistically significant (Table 1). Organisms isolated from the contaminated samples were Acinetobacter species, Klebsiella species, Bacillus species, Coagulase-negative Staphylococcus, E Coli, Micrococcus species and Pseudomonas aeruginosa, in which Coagulase-negative Staphylococcus (38.5%) was found to be more compared to other organisms (Table2). However majority of isolated organism (Table 3) were non-pathogenic (53.8%, p>0.05). Samples of GIC types collected in this study were Type I, II and III and among these types (Table 4), samples from Type I and III showed greatest bacterial contamination (83.3%) but was not statistically significant (p=0.525).
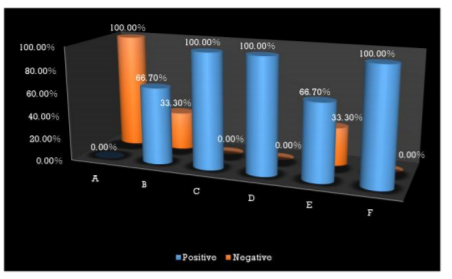
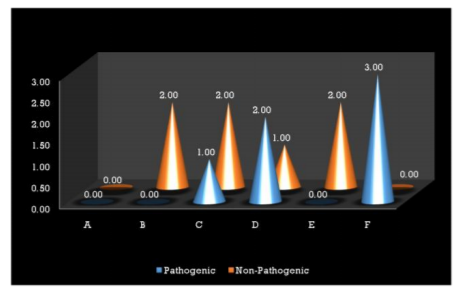
Interestingly most of the organisms were isolated from the Type I and Type III GIC samples (Figure 1), but the pathogenicity was more among Type III GIC samples (60%, p = 0.824) compared to other GIC types but was not statistically significant (Figure 2). As the samples were collected from 5 different dental setups, samples from B, C, D, E and F showed bacterial contamination (Figure 3) as compared to Sample A. But contamination was more among samples collected from C, D and F compared to others (p = 0.18). Among the contaminated samples of GIC collected from different dental setups, the pathogenic organism was more among F (100%), followed by D (66.7%) which was statistically significant (p=0.36) (Figure 4).
4. Discussion
This study was done to analyse aerobic bacterial contamination of Glass-ionomer cement in various dental setups. The microbial contamination of Glass-ionomer cement was fairly high in two satellite centres and mobile dental van as compared to other dental setups. Even though many aerobic bacteriae were isolated from clinically used containers. Staphylococcus species showed a direct or indirect involvement in the contamination of the dental setup. The reason may be the frequent use of the GIC with improper handling of the mixing pads which would result in generating numerous airborne infections. However, most such contamination occurs during its clinical usage. As the cement is manipulated on a mixing pad containing 34 sheets, a spatula is used to rapidly incorporate the powder into the liquid for the duration of 45–60 seconds during which there is a high chance of GIC getting contaminated. This contamination harbour microorganism, which later results in infectious disease to dental professionals and patients through cross-contamination.
The pads used to mix the cement are compressed paper on a cardboard base and these are commonly used to mix cement and bases. The porous nature of the cards can lead to contamination. About 34 sheets may be present as a bulk on first using, so when using the first sheet, chances are that it may seep through and contaminate the sheets below it. The mixing pad cannot be disinfected nor can a barrier be used between each sheet. After mixing and delivering, the used sheet is disposed and the remaining pad is kept in the drawer accompanied by the still remaining remnants of cement, materials or cotton rolls or they may be left out on the counter where they are easily contaminated.
Such contamination from mixing pad was later reported and revealed colonies between 1and 296 of Bacillus and gram-positive cocci[15]. In contrast, the present study showed colony count between 100 and 8000 colonies from different dental setup which was higher compared to the reported one. Airborne or salivary transmission of infections associated with GIC can affect dental professionals and patients in a dental setup. In addition, dental setup containing opportunistic pathogens should also be considered hazardous for immune suppressed patients, who could develop serious infections.
Microbial contamination of dental cement was higher in the range of 100-8000 CFU/m3 compared to previous studies[8–11,14]. Although those studies were done in factory sealed containers, the present study was carried out in containers that have been in clinical use and this could be one of the reasons for the rise in CFU in the present study. Among the types of GIC, samples from Type I and III showed increased bacterial contamination, these could be due to the increased application during dental procedures.
The risk associated with such contamination is high and measures need to be considered to reduce this contamination. Therefore this has to be taken into serious consideration by the Health Department, SPICE, Occupational Safety & Health Administration (OSHA), U.S. Food and Drug Administration (FDA), Centres for Disease Control and Prevention (CDC), and Healthcare Infection Control Practices Advisory Committee (HICPAC). The CDC recommends Guidelines for Infection Control in Dental Health-Care Settings — 2003. According to it, the dental health care professionals can be exposed to various pathogenic microorganisms including the CytoMegalo Virus (CMV), HBV, HCV, HSV types I, II, HIV. Mycobacterium tuberculosis, Staphylococci, Streptococci, other bacteria and viruses which infect or colonize the oral cavity and respiratory tract. The transmission of these microorganisms in dental settings occurs through 1) direct contact with blood, oral fluids, or other patient materials; 2) indirect contact with contaminated objects (e.g., instruments, equipment, or environmental surfaces; 3) contact of conjunctival, nasal, or oral mucosa with droplets (e.g., splatter) containing microorganisms generated from an infected person and propelled a short distance (e.g., by coughing, sneezing, or talking); and 4) inhalation of airborne microorganisms that can remain suspended in the air for long periods. According to CDC, Exposing patients or dental health-care personnel to water of uncertain microbiological quality, despite the lack of documented adverse health effects, is inconsistent with generally accepted infection control principles, and this statement is applicable to use of the dental mixing pads. Hence more stress should be given to focusing on infection control guidelines while handling GIC mixing pads.
5. Conclusion
The focal point of concern when considering the increasing incidence of GIC contamination in dental setups is poor infection control, which had its roots in the manipulation procedure. These issues can be handily dealt with, through certain specific courses of action that include a meticulous attention to details related to proper infection control, and the introduction of dental mixing sheet dispensers. Nevertheless, the viability of these suggestions requires further, in-depth studies, that when proved beyond doubt to be acceptable solutions, must certainly be put into action. Health is the prime objective of dental professionals and the patients they treat, it is mandatory that the centres responsible for the achievement of an excellent health status, do their part in ensuring the promotion of health. This includes the initiation of preventive protocol specific to contamination via the clinical usage of dental materials. When serious measures are taken, the significant ground is covered and superior results are obtained.
References
- Singh A, Purohit BM, Bhambal A, Saxena S, Singh A, Gupta A. Knowledge, attitudes, and practice regarding infection control measures among dental students in Central India. J Dent Educ 2011; 75(3):421–7.
- Bell DM. Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview. Am J Med 1997; 102(5B):9–15.
- Henderson DK, Fahey BJ, Willy M, Schmitt JM, Carey K, Koziol DE, et al. Risk for occupational transmission of human immunodeficiency virus type 1 (HIV-1) associated with clinical exposures. A prospective evaluation. Ann Intern Med 1990; 113(10):740–6.
- Margolis HS, Alter MJ, Hadler SC. Viral hepatitis. In: Evans AS, Kaslow RA, eds. Viral infections of humans, 4thed. New York: Plenum Medical Books Co; 1997. P.363–418.
- Esteban JI, Sauleda S, Quer J. The changing epidemiology ofhepatitis C virus infection in Europe. J Hepatol2008; 48:148–62.
- Verrusio AC, Neidle EA, Nash KD, Silverman S, Horowitz AM, Wagner KS. The dentist and infectious diseases: a national survey of attitudes and behavior. J Am Dent Assoc 1989; 118(5):553–62.
- Girdler NM, Matthews RW, Scully C. Use and acceptability of rubber gloves for outpatient dental treatment. J Dent 1987; 15(5):209–12. Ghavam M, Aligholi M. Bacterial contamination of four commonly used dental materials. Journal of Islamic Dental Association of Iran 2006; 18(3):84–91.
- Casemiro LA, Martins CH, de Souza Fde C, Panzeri H, Ito IY. Bacterial, fungal and yeast contamination in six brands of irreversible hydrocolloid impression materials. Braz Oral Res 2007; 21(2):106–11.
- Sadeghi M, AssarS.Microbial Contamination of 19 Consumable Dental Materials: An In Vitro Study .J Mash Dent Sch 2010; 34(2):135–42.
- Guru R, Saleem M, Singh R, Patil A. Microbiological appraisal of three different brands of commercially available irreversible hydrocolloid impression materials: an in vitro study. J Contemp Dent Pract 2011; 12(1):35–40.
- Anusavice K, Shen C, Rawls HR. Phillips' Science of Dental Materials, 11th ed. United Kingdom: Elsevier Health Sciences; 2012.
- McCabe JF, Walls AWG. Applied Dental Materials, 9th ed. Copenhagen: Blackwell Munksgaard; 2013.
- Rice CD, Moghadam B, Gier RE, Cobb CM. Aerobic bacterial contamination in dental materials. Oral Surg Oral Med Oral Pathol1990; 70(4):537–9.
- Smith DM. A solution to cross-contamination in the dental practice. http://www.dentistry q.com/articles/2013/06/a-solution-to-cross-contamination-in-the-dental-practice.html (Accessed 11 December 2016).