Information
Journal Policies
Efficacy of Plasma Activation on Bleaching
Fatma Yilmaz1, Esra Uzer Celik2*, Utku Kursat Ercan3, Fatma Ibis4
2.Department of Restorative Dentistry, Faculty of Dentistry, Izmir Katip Celebi University, Aydınlıkevler Campus, 35640 Cigli, Izmir, Turkey
3.Department of Biomedical Engineering, Faculty of Engineering and Architecture, Izmir Katip Celebi University, Cigli Main Campus, 35620 Cigli, Izmir, Turkey
4.Department of Biomedical Engineering, Faculty of Mechanical, Maritime and Materials Engineering, Delft University of Technology, Mekelweg, 2628, CD Delft, Nederlands
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Objectives: This in vitro study aimed to evaluate the bleaching efficacy and color stability of nonthermal atmospheric pressure plasma (NAPP)-activated 40% hydrogen peroxide (HP) and deionized water (DW). Methods: Two hundred-forty bovine enamel−dentin samples were stained with a coffee solution before bleaching. Samples were randomly divided into 12 (n = 20): (1) HP for 5 minute (HP-5), (2) HP-10, (3) HP-20, (4) Plasma-HP-5, (5) P-HP-10, (6) P-HP-5/HP-15, (7) P-HP-10/HP-10, (8) P-DW-5, (9) P-DW-10, (10) DW-5, (11) DW-10, and (12) DW-20. After bleaching, the samples were re-stained for 48 h using red wine. The color differences measured using a dental spectrophotometer after bleaching and after re-staining were reported as ΔE1 and ΔE2, respectively. The data were analyzed using Kruskal−Wallis and Mann−Whitney U tests (p< 0.05).
Results: All plasma-activated groups revealed higher ΔE1 values than the control groups and similar or higher ΔE1 values than the nonactivated HP groups (p0.05). The HP-5 and HP-10 groups showed lower ΔE2 value than the other groups (p< 0.05), and ΔE2 value of the HP-20 group was similar to the other plasma-activated groups.
Conclusion: Nonthermal atmospheric pressure plasma treatment increased the bleaching efficacy of HP and gave similar bleaching efficacy of HP to DW with similar color stability.
Keywords: Nonthermal atmospheric pressure plasma, vital bleaching, hydrogen peroxide, deionized water,Dental Science
1. Introduction
Today, light sources such as halogen light, light-emitting diodes, and laser are widely used for the in-office bleaching technique to hasten the whitening process[1]. Although, the main role of light source is controversial [2,3], light is considered to increase bleaching efficacy by increasing the accumulation of oxygen in free radicals [4]. Aside from its debatable effectiveness, the light sources may raise the temperature of the tooth and generate thermal-irreversible adverse effects on the pulp and enamel surface if it is not used in the right manner [5,6].
In recent years, nonthermal atmospheric pressure plasmas (NAPP) have been reported as an activator in dental bleaching [7-9], as they do not transfer excessive heat to the tooth surface during bleaching. Plasma is the fourth state of matter and is a highly reactive material consisting of charged particles, radicals, and a strong electric field. In previous studies [8,10-14], NAPP jet increased the bleaching efficacy of HP and carbamide peroxide (CP) by producing energetic ions, free electrons, and hydroxyl radicals [15]. However, the jet system was found impractical because gas flow led to the removal of gel from the enamel surface.
In a previous study, a hybrid gas−water plasma achieved tooth whitening[9]. During the preliminary study, we also noticed that when we had applied NAPP onto wet discolored teeth, a bleaching process was stimulated and teeth became lighter. After this observation, we speculated that deionized water (DW) could be a transfer (or diffusion) medium of plasma-generated species for tooth bleaching [16].
The aim of this in vitro study to examine the bleaching efficacy and color stability of the NAPP-activated HP and DW. In this study, nonthermal, atmospheric pressure dielectric barrier discharge (DBD) air plasma was used instead of plasma jet to eliminate removal of bleaching agents from the enamel surface. The null hypothesis of this study is that “no difference would be found in bleaching efficacy and color stability between plasma-activated and nonactivated agents”.
2. Materials And Methods
The study protocol was approved by the Ethical Committee of the Faculty of Medicine, XXXXX (#2015-215). The enamel samples for bleaching were prepared in the Research Laboratory of the Faculty of Dentistry. Bleaching procedures were performed in the Medical Plasma Laboratory of the Faculty of Engineering and Architecture at XXXXX University.
A preliminary study was performed to determine the most effective plasma exposure time. For this purpose, 40% HP gel and DW were activated for 3, 5, 7, and 10 min with NAPP, and their bleaching effectiveness was compared with inactivated 40% HP. In light of the results, a significant increase was observed in bleaching efficacy of the plasma-activated group for 5 min compared with that of nonactivated 40% HP. For this reason, 5 and 10 min plasma exposure times were tested to increase the bleaching efficacy of HP and to give a bleaching effect to DW.
In this study, 120 extracted bovine incisors were used. The crowns were cut with a slow-speed diamond cutting saw (Isomet Diamond Wafering Blades, Buehler, USA) in the mesio-distal direction under water cooling. The buccal surfaces of each crown were used. Then, the buccal surfaces were divided into two in the bucco-palatal direction and 240 samples were obtained [17].
Before bleaching, the teeth were submitted to a discoloration process [18]. Dentin surfaces of the samples were etched with 37% phosphoric acid gel (Scotchbond Acid, 3M ESPE, St. Paul, MN, USA) for 15 s to open the dentinal tubules. The samples were kept in an incubator in 25% coffee mixture at 37 °C for one week. One week later, the samples were washed and dentinal tubules were sealed by two layers of transparent nail varnish. After this procedure, enamel surfaces were polished to eliminate the external discoloration. This process was completed by storing the discolored samples in artificial saliva for 14 days [19]. The saliva was refreshed every day.
The samples were divided into 12 groups, with 20 samples in each group, according to the bleaching methods and their application periods (n=20).
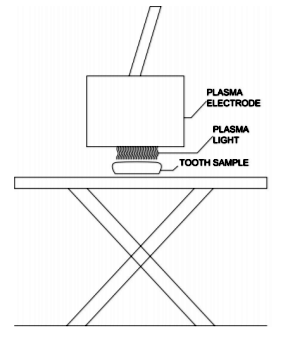
In the present study, all plasma treatments were performed using an alternating current (AC), microsecond-pulsed power supply. Nonthermal, atmospheric pressure DBD air plasma was generated at a frequency of 1.5 kHz, 22 kV of peak-to-peak voltage, and 5 µs of pulse duration by maintaining 2 mm of discharge gap (Figure 1).
Group 1 (HP-5): 40% HP gel (Opalescence® Boost PF 40%, Ultradent Inc., South Jordan, UT, USA) was applied for 5 min according to the manufacturer’s instructions. A 0.5−1.0 mm-thick layer of gel was applied to the labial surfaces. Samples with HP on them were held in an incubator for 5 min during application at 37 °C.
Group 2 (HP-10): The same procedures as those used in group 1 were applied but for 10 min.
Group 3 (HP-20): The same procedures as those used in group 1 were applied but for 20 min.
Group 4 (P-HP-5): 40% HP was applied to the samples, and they were then treated for 5 min with nonthermal atmospheric DBD plasma at the previously described parameters.
Group 5 (P-HP-10): In this group, the same procedure in group 4 was performed but for 10 min.
Group 6 (P-HP-5/HP-15): After 40% HP was applied to the samples, it was activated by plasma performed as describe above for 5 min. Following the 5 min activation by plasma, the gel was not removed from the outer surface of the samples. Then, the samples were kept in an incubator at 37° C for 15 min.
Group 7 (P-HP-10/HP-10): Plasma was performed as describe above for 10 min. Following the 10 min activation by plasma, the gel was not removed from the outer surface of the samples. Then, the samples were kept in an incubator at 37° C for 10 min.
Group 8 (P-DW-5): DW was applied to the samples and then activated by plasma as describe above for 5 min. However, to ensure the electric field, the plasma source was switched off once every 1 min and then 1 drop of DW was placed onto the buccal surfaces of samples.
Group 9 (P-DW-10): In this group, the same procedure in group 8 was performed but for 10 min.
Group 10 (control group) (DW-5): The samples were kept in a DW-filled container for 5 min in an incubator at 37 °C as a control group of HP-5, P-HP-5, and P-DW-5.
Group 11 (control group) (DW-10): The samples were kept in a DW-filled container for 10 min in an incubator at 37 °C as a control group of the HP-10, P-HP-10, and P-DW-10 groups.
Group 12 (control group) (DW-20): The samples were kept in a DW-filled container for 20 min in an incubator at 37 °C as a control group of the HP-20, P-HP-5/HP-15, and P-HP-10/HP-10 groups.
After each application, the samples were cleaned with a brush under water for 30 s and evaluated. Following the completion of the bleaching process, the samples were polished using polishing discs (Sof-LexTM Polishing Discs, 3M ESPE, St. Paul, MN, USA) under water cooling. Then, fluoride gel (Elmex Gel, Gaba, Lörrach, Germany) was applied to the samples for 4 min.
The samples were kept in artificial saliva for 14 days after bleaching had been completed and subjected to red wine (Kaplankaya Wines, Sirince, Izmir, Turkey) for 48 h [20]. Following the re-staining process, the samples were cleaned by a toothbrush (Oral-B Indicator Plus 35, Gillette do Brasil Ltda., Manaus, Amazonas, Brazil) with a nonabrasive toothpaste (Colgate Total 12, Colgate-Palmolive Ltda., Osasco, São Paulo, Brazil). External discoloration was removed by pumice and polishing rubber cups.
Vita colors and L*a*b values of the samples were measured by a dental spectrophotometer (Spectroshade, MHT OpticReasearch AG, Niederhasli, Switzerland) after discoloration, bleaching, and re-staining. Measurements were performed in a dark room and repeated three times, but the average value was used for further analysis. The color changes occurring after bleaching (ΔE1) and after re-staining (ΔE2) were calculated by the following equation: ΔE1 = ((L2-L1)2+(a2-a1)2+(b2-b1)2)1/ 2 L1, a1, and b1: L, a, and b values after staining, L2, a2, and b2: L, a, and b values after bleaching; ΔE2 = ((L3-L2)2+(a3-a2)2+(b3-b2)2)1/2(L3, a3, and b3: L, a, and b values after re-staining, L2, a2, and b2: L, a, and b values after bleaching).
Three human central incisors were used to measure tooth temperature in each plasma-activated group. The temperature increase was measured by a fiber optic temperature measurement system (FTI-10 fiber optic signal conditioner, FOT-L-SD fiber optic temperature sensor; FISO Technologies Inc., Québec, Canada). The measurements were performed immediately after the bleaching process. The fiber optic temperature sensor was placed in contact with the buccal surface, and the tooth was properly fixed. The measurements were performed on each tooth at room temperature (25 °C).
Statistical analysis was conducted using the SPSS software system version 20.0 (IBM Corporation, New York, USA). Confidence analysis was performed by the Kolmogorov −Smirnov test (df=40), which proved that the values were not normally distributed. The Kruskal−Wallis test was used to make multiple comparisons of the groups. Binary comparisons within the group were performed by the Mann−Whitney U test. The results were evaluated at the 95% confidence interval and at the p < 0.05 level.
3. Results And Discussion
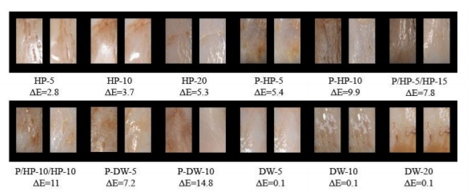
Examples of each group taken before and after bleaching are given in Figure 2.
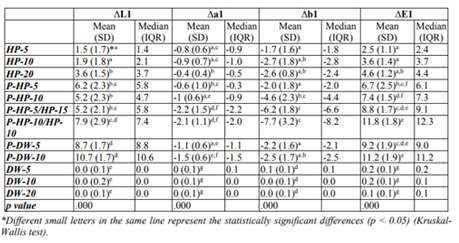
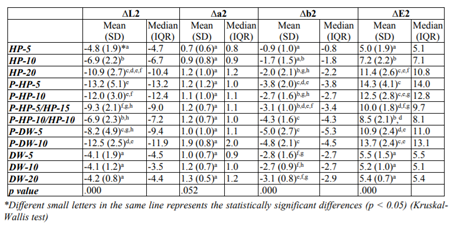
Color difference values of the tested bleaching methods and their statistical comparison results are given in Table 1 and 2.
Color improvement after bleaching (ΔE1 value) in the experimental groups was significantly higher than that in the control groups (p < 0.05). No statistical differences were observed among ΔE1 values of the control groups. In the experimental groups, the HP-5 and HP-10 groups revealed lower ΔE1 values than the other groups (p < 0.05). No differences were found in the ΔE1 values of the HP-20 and P-HP-5 groups, and the HP-20 group showed a lower ΔE1 value than that of the other plasma-activated groups (p < 0.05).
In the plasma-activated groups, the ΔE1 values of the P-HP-5 and P-HP-10 groups were lower than those of the P-DW-10 and P-HP-10/HP-10 groups (p < 0.05). No significant differences were observed in the other pairwise comparisons.
Re-staining after bleaching (ΔE2 values) in all of the experimental groups were significantly higher than those of the control groups except for the HP-5 group (p < 0.05). No statistical differences were observed in the control groups in terms of ΔE2 values. In the experimental groups, the HP-5 and HP-10 groups showed lower ΔE2 than the other groups (p < 0.05). The ΔE2 value of the HP-20 group was similar to those of the plasma activated groups.
In the plasma activated groups, the P-HP-10/HP-10 group showed statistically less re-staining than the P-HP-5, P-HP-10, P-DW-5, and P-DW-10 groups (p < 0.05). The ΔE2 value of the P-DW-5 group was also statistically lower than that of the P-HP-5 group (p < 0.05).
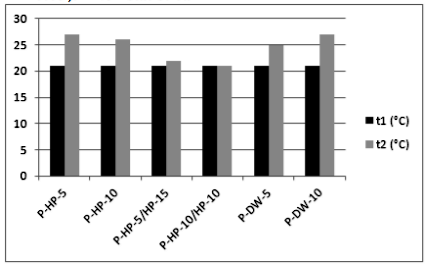
In the plasma-activated groups, temperature values before (t1) and after (t2) applications are given in Figure 3. The temperature did not increase to the intraoral temperature of 35° C−36° C in any of the groups [21].
According to the findings, the null hypothesis that “no difference would be found in bleaching efficacy and color stability between plasma-activated and nonactivated agents” was rejected. In the present study, NAPP increased the bleaching efficacy of HP and shortened the application time of the conventional in-office bleaching technique without light activation. The P-HP-5 group revealed 2.5 times more effective bleaching efficacy than HP-5. Similarly, the P-HP-10 group showed two times more effective bleaching than HP-10. We could not apply the plasma for 20 min because a long application time could cause excessive heat on the electrode surface that could lead to the corruption of the DBD electrode. However, we obtained similar bleaching efficacy in the HP-20 group when we applied 40% HP gel with plasma for 5 min (P-HP-5) and obtained better results than those of the HP-20 group when we applied 40% HP gel with the other plasma application modes (P-HP-10, P-HP-5/HP-15, and P-HP-10/HP-10).
In previous in vitro studies, NAPP was also used as a light source to increase the bleaching efficacy of HP and CP, as it is a nonthermal and nontoxic method [7,8, 22]. Park,et al. [23] reported that the NAPP jet enhanced the bleaching efficacy of 30% HP in intracoronal tooth bleaching. Nam et al. [24] observed that the NAPP jet-activated 16% CP was 3.7 times more effective than its nonactivated counterpart in tooth bleaching. They also compared the bleaching effectiveness of 16% CP activated by three different light sources (i.e., NAPP jet, PAC, and diode laser) and reported that the highest ∆E value was reached using the NAPP jet [25]. However, the plasma source used in these previous studies was different from the device used in the present study. We preferred the DBD air plasma over the NAPP jet as the jet system removes the gel away from the surface because of the high speed gas flow and the gel requires replacement at certain intervals [23,26]. Helium gas at a flow rate of 2 L min-1 is commonly used as feeding gas though the NAPP jet device. If helium is present even in just a small amount in the atmosphere, it changes the atmospheric conditions of the working environment. Moreover, inhaling helium can be dangerous because it is a simple asphyxiant and may displace oxygen needed for normal respiration [27].
Atomic oxygen radicals, which are highly reactive species, play an important role in the biomedical applications of plasma [28,29]. Plasma treatment provides bleaching by uncovering back the colorant organic molecules that are typically maintained on the enamel surface and by removing or denaturing proteins [13]. Furthermore, plasma may be effective in tooth bleaching by breaking the linked pairs of pigmented protein chains and modifying them to a single bond through the aid of OH ions released from plasma and quickly diffused to the enamel surface. Therefore, plasma creates a synergistic mechanism with bleaching agents and accelerates the bleaching process.
NAPP activation produced an effective tooth bleaching effect when it was applied with DW in the present study. The P-DW-5 group revealed 3.7 times more effective bleaching efficacy than the HP-5 group; similarly, the P-HP-10 group showed 3.1 times more effective bleaching than the HP-10 group. Furthermore, we revealed a better bleaching effect when we applied DW to all plasma activation modes (the P-DW-5, P-DW-10 groups) compared with the HP-20 group. In addition, the P-DW-10 group showed a superior bleaching effect with the P-HP-10/HP- 10 group to other plasma-activated groups. Similarly, tooth stain was removed within 10 min by a hybrid gas−water plasma in a previous study [9].
The effective bleaching effect of plasma-treated DW may be explained by the formation of reactive oxygen species, charged particles, and oxidizing radicals that are stronger than ozone [30, 31]. Both HP and OH radicals are generated after energetic electrons have interacted with the water molecules. OH radicals have been speculated to be mainly in charge of the tooth whitening process by the hybrid gas−liquid plasma [9]. In addition, plasma-activated DW has also been reported to possess an acidic feature [9, 32]. Shainsky et al. [33] showed that although the HP concentration in DW increased to 2000 mg/L at the beginning of the plasma application, it decreased to 10 mg/L after a 15 min application.
The oxidation ability of HP is high at low pH values [34]. Furthermore, superoxide anions occur in DW after plasma treatment. The oxidizing property of HP is enhanced by superoxide anions [33]. This information may explain the effective bleaching effect of plasma-activated DW.
It has been reported that enamel permeability increases in proportion to the concentration of bleaching gel [35]. The increased permeability of enamel may accelerate re-staining. In the present study, ΔE2 values of the experimental groups were statistically higher than those of the control groups except for the HP-5 group. This group showed similar ΔE2 values with the control groups probably because of its short application time. Similarly, bleached enamel by 35% HP re-stained more than nonbleached enamel [20, 36, 37]. Plasma activation of HP and DW for 5 min and 10 min revealed more re-staining than their nonactivated counterparts. The reason for this finding may be attributed to their effective bleaching effect. In previous studies, bleached teeth with high color difference values were also prone to re-staining [38, 39]. However, all plasma-activated groups produced similar re-staining with HP-20. This finding may indicate that plasma activation increases bleaching activity without any irreversible damage to the composition and surface morphology of enamel. In previous studies [36, 40], susceptibility to re-staining of enamel after bleaching depended on surface roughness, chemical composition, and dehydration amount occurring after the bleaching process.
In the present study, enamel surface morphology reached a maximum of 27°C after NAPP application. Similarly, the NAPP jet revealed no extra increase in temperature compared with the nonactivated bleaching groups or other light sources.[24-26] Sulieman et al. [41] claimed that bleaching gel and its water content act as a barrier preventing the increase in pulp temperature caused by light sources.
4. Conclusions
In this study showed that NAPP treatment increased the bleaching efficacy of HP and shortened the bleaching period of the conventional in-office bleaching technique without light activation. In addition, plasma treatment gave a similar bleaching efficacy of HP to DW with similar color stability to re-staining.
Acknowledgements
This work was funded by the Scientific Research Projects Council at Izmir Katip Celebi University (grant number 2016-ÖDL-SABE-0005). The authors declare that they have no conflict of interest.
References
- Buchalla W. and Attin T., External bleaching therapy with activation by heat, light or laser-a systematic review, Dent. Mater. 23, 586-596 (2007).
- Tavares M., Stultz J., Newman M., Smith V., Kent R., Carpino E. and Goodson J. M., Light augments tooth whitening with peroxide, J. Am. Dent. Assoc. 134, 167-175 (2003).
- Luk K., Tam L. and Hubert M., Effect of light energy on peroxide tooth bleaching, J. Am. Dent. Assoc.135, 194-201 (2004).
- Yazici A. R., Khanbodaghi A. and Kugel G., Effects of an in-office bleaching system (ZOOM) on pulp chamber temperature, J. Contemp. Dent. Pract. 8, 19-26 (2007).
- Hein D. K., Ploeger B. J., Hartup J. K., Wagstaff R. S., Palmer T. M. and Hansen L. D., In-office vital tooth bleaching--what do lights add?, Compend. Contin. Educ. Dent. 24, 340-352 (2004).
- Pinto C. F., Oliveira Rd., Cavalli V. and Giannini M., Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology, Braz. Oral. Res. 18, 306-311 (2004).
- Iza F., Lee J. K.and Kong M.G., Electron kinetics in radio-frequency atmospheric-pressure microplasmas, Physical review letters http://http://journals.aps.org/10.1103/PhysRev Lett.99.075004 doi:10.1103/. 99.075004(2007 ).
- Iza F., Kim G. J., Lee S. M., Lee J. K., Walsh J. L., Zhang Y. T. and Kong M. G., Microplasmas: Sources, particle kinetics, and biomedical applications, Plasma Processes Polym. 5, 322-344 (2008).
- Kim S. M., Koo G. I., Choi M. Y., Jung J. C., Eldali F., Lee J. K. and Collins G. J., Corralated electrical and optical studies of hybrid argon gas–water plasmas and their application to tooth whitening, Plasma Process. Polym. 9, 339-345 (2012).
- Kieft I. E., Broers J. L., Caubet-Hilloutou V., Slaaf D. W., Ramaekers F. C. and Stoffels E., Electric discharge plasmas influence attachment of cultured CHO K1 cells, Bioelectromagnetics 25, 362-368 (2004).
- Park G., Lee H., Kim G. and Lee J. K., Global model of He/O2 and Ar/O2 atmospheric pressure glow discharges, Plasma Process. Polym. 5, 569-576 (2008).
- Laroussi M., Low-temperature plasmas for medicine?, IEEE T. Plasma Sci. 37, 714-725 (2009).
- Lee H. W., Kim G. J., Kim J. M., Park J. K., Lee J. K. and Kim G. C., Tooth bleaching with nonthermal atmospheric pressure plasma, J. Endod. 35, 587-591 (2009).
- Claiborne D., McCombs G., Lemaster M., Akman M. A. and Laroussi M., Low‐ temperature atmospheric pressure plasma enhanced tooth whitening: the next‐generation technology, Int. J. Dent. Hyg. 12, 108-114 (2012).
- M. Laroussi, M. Kong, G. Morfill and W. Stolz, Plasma medicine: Applications of low-temperature gas plasmas in medicine and biology, 1st ed. New York, U. S.: Cambridge UniveWSrsity Press, 2012, ch. 2, pp: 7-28.
- Kim G. C., Lee H. W., Byun J. H., Chung J., Jeon Y. C. and Lee J. K., Dental applications of low‐temperature nonthermal plasmas, Plasma Processes Polym. 10, 199-206 (2013).
- D'Arce M., Lima D., Aguiar F. H., Ambrosano G. M., Munin E. and Lovadino J. R., Evaluation of ultrasound and light sources as bleaching catalysts-an in vitro study, Eur. J. Esthet. Dent. 7, 176-184 (2012).
- Batista G., Barcellos D., Torres C., Goto E., Pucci C. and Borges A.B., The influence of chemical activation on tooth bleaching using 10% carbamide peroxide, Oper. Dent. 36, 162-168 (2011).
- Leung V. W. and Darvell B.W., Artificial salivas for in vitro studies of dental materials., J. Dent. 25, 475-484 (1997).
- Berger S. B., Coelho A. S., Oliveira V. A. P., Cavalli V. and Giannini M., Enamel susceptibility to red wine staining after 35% hydrogen peroxide bleaching., J. App. Oral Sci. 16, 201-204 (2008).
- Moore R. J., Watts J. T. F., Hood J. A. A. and Burritt D. J., Intra-oral temperature variation over 24 hours, Eur. J. Orthodont. 21, 249-261 (1999).
- Lu X., Jiang Z., Xiong Q., Tang Z. and Pan Y., A single electrode room-temperature plasma jet device for biomedical applications, Appl. Phys. Lett. 92(15), 151504 (2008).
- Park J. K., Nam S. H., Kwon H. C., Mohamed A. A., Lee J. K. and Kim G. C., Feasibility of nonthermal atmospheric pressure plasma for intracoronal bleaching, Int. Endod. J. 44, 170-175 (2011).
- Nam S. H., Lee H. W., Cho S. H., Lee J. K., Jeon Y. C. and Kim G. C., High-efficiency tooth bleaching using non-thermal atmospheric pressure plasma with low concentration of hydrogen peroxide, J. App. Oral Sci. 21, 265-270 (2013).
- Nam S. H., Lee H. J., Hong J. W. and Kim G. C., Efficacy of nonthermal atmospheric pressure plasma for tooth bleaching, ScientificWorld Journal 581731, (2015).
- Sun P., Pan J., Tian Y., Bai N., Wu H., Wang L., Yu C., Zhang J., Zhu W. and Becker K. H., Tooth whitening with hydrogen peroxide assisted by a direct-current cold atmospheric-pressure air plasma microjet, IEEE T. Plasma Sci. 38, 1892-1896 (2010).
- Grassberger M. and Krauskopf A., Suicidal asphyxiation with helium: Report of three cases, Wien. Klin. Wochenschr. 119, 323-325 (2007).
- Laroussi M., Low temperature plasma‐based sterilization: overview and state‐of‐the‐art, Plasma Process. Polym. 2, 391-400 (2005).
- Kim S. J., Chung T. H., Bae S. H. and Leem S. H., Bacterial inactivation using atmospheric pressure single pin electrode microplasma jet with a ground ring, Appl. Phys. Lett. 94(14), 141502 (2009).
- Burlica R., Kirkpatrick M. J. and Locke B. R., Formation of reactive species in gliding arc discharges with liquid water, J. Electrostat. 64, 35-43 (2006).
- Brisset J. L., Moussa D., Doubla A., Hnatiuc E., Hnatiuc B., Youbi G. K., Herry J. M., Naïtali M. and Bellon Fontaine M. N., Chemical reactivity of discharges and temporal post-discharges in plasma treatment of aqueous media: examples of gliding discharge treated solutions, Ind. Eng. Chem. Res. 47, 5761-5781 (2008).
- Ikawa S., Kitano K. and Hamaguchi S., Effects of pH on bacterial inactivation in aqueous solutions due to low-temperature atmospheric pressure plasma application, Plasma Process. Polym. 7, 33-42 (2010).
- Shainsky N., Dobrynin D., Ercan U., Joshi S., Ji H., Brooks A., Fridman G., Cho Y., Fridman A. and Friedman G., Non-equilibrium plasma treatment of liquids, formation of plasma acid, International Symposium on Plasma Chemistry (ISPC 20), Philadelphia, 1–4 (2011).
- C. W. Jones, Applications of hydrogen peroxide and derivatives, 1st ed. Widnes, UK: The Royal Society of Chemistry, 1999, ch. 1, pp:1-36.
- Malkondu Ö., Yurdagüven H., Say E. C., Kazazoglu E. and Soyman M., Effect of bleaching on microhardness of esthetic restorative materials, Oper. Dent. 36, 177-186 (2011).
- Rotstein I., Dankner E., Goldman A., Heling I., Stabholz A. and Zalkind M., Histochemical analysis of dental hard tissues following bleaching, J. Endod. 22, 23-25 (1996).
- Zalkind M., Arwaz J., Goldman A. and Rotstein I., Surface morphology changes in human enamel, dentin and cementum following bleaching: a scanning electron microscopy study, Dent. Traumatol. 12, 82-88 (1996).
- Setien V., Roshan S., Cala C. and Ramirez R., Pigmentation susceptibility of teeth after bleaching with 2 systems: an in vitro study, Quintessence Int. 40, 47-52 (2009).
- Alaghemand H., Kamangar S. S. H., Zarenegad N., Tabari N., Abedi H. and Khafri S., In-vitro effect of casein phosphopeptide amorphous calcium phosphate on enamel susceptibility to staining by tea during bleaching treatment, J. Dent. 12, 607–613 (2015).
- Titley K., Torneck C. D. and Smith D., The effect of concentrated hydrogen peroxide solutions on the surface morphology of human tooth enamel, J. Endod. 14, 69-74 (1988).
- Sulieman M., Rees J. and Addy M., Surface and pulp chamber temperature rises during tooth bleaching using a diode laser: a study in vitro, Br. Dent. J. 200, 631-634 (2006).