Information
Journal Policies
Study on the Effect of Ethanol Extract of Ipomoea Aquatica (Kalmi Shak) Leaves on Gentamicin Induced Nephrotoxic Rats
Dr. Rayhana Sharmin. MBBS, M Phil. (Pharmacology)1, Dr. A.B.M.Iftekhar Hossain, MBBS. (Pathology) 2, Dr. Sharmin Rahman. MBBS, M Phil. (Pharmacology) 3, Dr. Azmary Momtaz. MBBS, M Phil. (Pharmacology) 4, Dr. Khaleda Sharmin. MBBS, M Phil. (Pharmacology) 5, Dr. Abu Syed Md. Mosaddek, MBBS, M Phil. (Pharmacology)6
2.Department of Pathology, Mymensingh Medical College, Bangladesh.
3.Assistant Professor, Department of Pharmacology and Therapeutics, Ibrahim Medical College, Dhaka, Bangladesh.
4.Assistant Professor, Department of Pharmacology and Therapeutics, Delta Medical College, Dhaka, Bangladesh.
5.Assistant Professor, Department of Pharmacology and Therapeutics, University Dental College, Dhaka, Bangladesh.
6.Professor and head, Department of Pharmacology and Therapeutics, Uttara Adhunik Medical College, Dhaka, Bangladesh.
Copyright : © 2016 Sharmin R. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Drug-induced kidney disease constitutes an important cause of acute renal failure. Recent studies indicate that free radicals are important mediators of kidney injury induced by gentamicin (GM), an aminoglycoside antibiotic widely used in treating severe gram-negative infection. Ipomoea aquatica (IA) was reported to have antioxidant and free redical scavenging activities.
Objective: The present study was performed to find out the nephroprotective effect of Water spinach IA ethanol extract leaves On Gentamicin-induced nephrotoxic rats.
Materials and methods: The experiment was carried out in the department of Pharmacology of Dhaka Medical College, Dhaka, from January 2013 to December 2013. For the purpose of the study 32 wistar albino rats either both sex were taken and divided into four groups (Gr-I, Gr-II, Gr-III and Gr-IV) consisting 8 rats each. Group I received laboratory diet and distilled water for 14 days. Group II received IA ethanol extract at the rate of 500 mg/kg body weight orally by gastric intubation for 14 days. Group III received normal diet for 14 days and injection gentamicin at a dose of 80 mg/kg/day intraperitoneal from day seven to day fourteen. Group IV received ethanol extract of Ipomoea aquatica 500 mg/kg/day orally from day one to day six,then received concomitant treatment with injection gentamicin at a dose of 80 mg/kg/day intraperitoneal from day seven to day fourteen.
Results: The serum creatinine and serum urea levels were significantly (p < 0.01) increased in rats treated with GM (Gr-III) as compared to control group. Histopathology of kidney of this group also showed massive interstitial nephritis. Again Pre & concomitant administration of IA ethanol extract on GM induced nephrotoxic rats (Gr-IV) significantly (p < 0.01) decreased the elevated serum creatinine and urea levels when compared to those of GM treated group. The comparative histopathological study of kidney exhibited almost normal architecture as compared to control group.
Conclusion: It may be concluded that, Ipomoea aquatica may protect and cure kidney tissues against nephrotoxic effect of gentamicin, though accurate mechanism and safety profile is not confirmed by this study.
Keywords: Gentamicin, Ipomoea Aquatic, Serum Creatinine, Serum Urea.
1.Introduction
Acute renal failure (ARF) refers to the sudden and usually reversible loss of renal function which develops over a period of days or weeks [1]. It is characterized by abrupt and sustained decline in glomerular filtration rate (GFR), which leads to accumulation of urea and other chemicals in the blood [2]. Metabolites of the drugs that are excreted through kidney cause cellular damage leading to kidney dysfunction [3].
Gentamicin is an important aminoglycoside antibiotic commonly used in treating life threatening gram-negative infections [4]. However its usefulness is limited by signs of nephrotoxicity, which may occur in 13-30% of treated patients [5]. Nephrotoxicity is a major complication of the gentamicin administration. Thus amelioration of nephrotoxicity would enhance its clinical use [6]. The mechanisms of gentamicin nephrotoxicity are not completely known. However the pathological mechanisms involved in gentamicin induced nephrotoxicity include induction of oxidative stress [7, 8]. Gentamicin has been showed to increase the generation of super oxide anions (O2-), hydroxyl radicals (OH-), hydrogen peroxide and reactive nitrogen species in kidney and lead to renal injuries [9]. In other hand, gentamicin reduces efficiency in kidney antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) and glutathione (GSH) [7].
Some antioxidant agents that have been used to ameliorate gentamicin induced nephrotoxicity in rats include deferoxamine, methimazole, vitamin E, vitamin C diethyl dithiocarbamate, L-histidinol, thymoquinone [10]. But none of these compounds have proved to be clinically efficient to provide complete protection in patients. Recently, interest has considerably increased in finding naturally occurring antioxidant that are able to ameliorate gentamicin induced nephrotoxicities, to replace synthetic antioxidants, which were restricted due to their side effects such as carcinogenesis [11]. In this study, we have explored the possible protective role of Ipomoea aquatica extract on gentamicin-induced renal injury.
Ipomoea aquatica Forsk (IA.) belongs to the botanical family Convolvulaceae, commonly known as kalmi shak or water-spinach; is a perennial herb found throughout Tropical Asia, Ceylon, Africa and Australia [12].Phytochemical screening of I.aquatica revealed high concentrations of alkaloids, reducing sugar, soluble carbohydrate, flavonoids, phenol, β-carotene, tannins etc. IA exhibits excellent antioxidant activity in its’ different extracts which includes O2- & OH- scavenging, iron chelation and electron donation. Both ethanol extract of leaf and stem had much higher amount flavonoid compounds than water extract. Ethanol extract of leaf had the highest flavonoid content. The antioxidant activity was directly related to the total amount of flavonoids found in the water spinach extracts [13].
It may be assumed that Ipomoea aquatica is cheap, easily obtainable, environmental friendly and lacks significant adverse effects. It would be beneficial to obtain its nephroprotective properties and then useful medicines could be obtained through research from this herbal agent.
In many countries, lots of work have been done on nephroprotective effect of many herbs and plants including Ipomoea aquatica. With this background information in this study attempt has been made to evaluate the nephroprotective effect of Ipomoea aquatica extract in experimental nephrotoxic rats. Gentamicin has been chosen to induce nephrotoxicity in rats. Serum creatinine & serum urea estimated to the extent of nephroprotective effect of Ipomoea aquatic extract in experimental nephrotoxic rats.
2. Materials and Methods
The study was performed in the department of Pharmacology at Dhaka medical college, Dhaka from January 2013 to December 2013.
A total of 32 healthy adult wistar albino rats weighing between 180-200gms, age 8-10 weeks were collected from the Bangladesh Centre for Scientific and Industrial Research (BCSIR) Lab. The chosen animals were housed at animal house in Dhaka Medical College and they were in standard size metallic cages in a well-ventilated room. The rats were allowed to live at room temperature with 12 hours of light and 12 hours dark schedule. They were fed normal rat diet and given water ad libitum, then they were randomly assigned to the following 4 equal groups (i.e. each = 8 rats).
The fresh leaves of I. aquatica were obtained from a local vegetable market of Dhaka. They were identified and authenticated from Bangladesh National Herbarium, Mirpur, Dhaka. The DACB accession number is 39511.
Ethanol extract was made in the Drug Research Laboratory of Center for Advanced Research of Sciences (CARS) of Dhaka University. 4 kg of Ipomoea aquatic leaves were cleaned and shed dried. Then it was crushed into coarse powder and soaked in 96% ethanol (5L) with continuous shaking (40rmp) for three days and filtered by filter paper. The ethanol extract was evaporated under vacuum rotator evaporator at 40-50° C degree temperature to obtain final deep green semisolid extract. A total of 30 gram extract was found in this way.
Group I: (Control group): They were received laboratory diet and distilled water for 14 days.
Group II: (IA extract group): They received ethanol extract of IA at the rate of 500 mg/kg/day orally by gastric intubation along with standard rat food for 14 days.
Group III: (GM group): They received normal diet for 14 days and injection gentamicin at a dose of 80 mg/kg/day intraperitonealy from day seven to day fourteen [14].
Group IV: (IA extract + GM): They received IA extract 500 mg/kg/day orally by gastric intubation from day one to day six. Then concomitant treatment with injection gentamicin at a dose of 80 mg/kg/day intraperitonealy and IA ethanol extract 500 mg/kg/day orally from day seven to day fourteen.
On 15th day the blood was collected by cardiac puncture. Then plasma was separated by centrifugation at 3000 rpm for 15 min. then stored at -70oC for estimation of serum creatinine and serum urea. Then rats were sacrificed under light anesthesia with chloroform and kidneys were preserved in 10% formalin for histopathological study.
Histological observations were made by the following way
Normal kidney: Normal glomeruli & proximal tubules and with normal interstitium and blood vessels.
Mild interstitial nephritis: Alleviation of renal architecture including glomeruli and tubules with interstitium infiltrated by moderate number of lymphocyte.
Massive interstitial nephritis: Glomerular congestion and necrosis in the proximal tubular. Interstitium showed infiltration with inflammatory cells.
Collected data were tabulated & statistical analysis was done by appropriate significant test. Student’s unpaired‘t’ test was used to compare the results between individual groups. Results were expressed as mean ± SD and values of probability P of ˂0.05 and P of ˂0.01 will be considered to be statistically significant and highly significant respectively.
3.Results
The effect of ethanolic extract of Ipomoea aquatica treatment on the Gentamicin- induced nephrotoxicity on serum creatinine and serum urea are shown on table (I). Administration of gentamicin (Group III) significantly (p < 0.01) elevated the level of serum creatinine and urea. Pre & concomitant administration of IA ethanol extract on GM induced nephrotoxic rats (Group IV) significantly (p < 0.01) decrease the elevated serum creatinine and urea levels. However only IA ethanol extract administration (Group II) had no significant effect on these parameters.
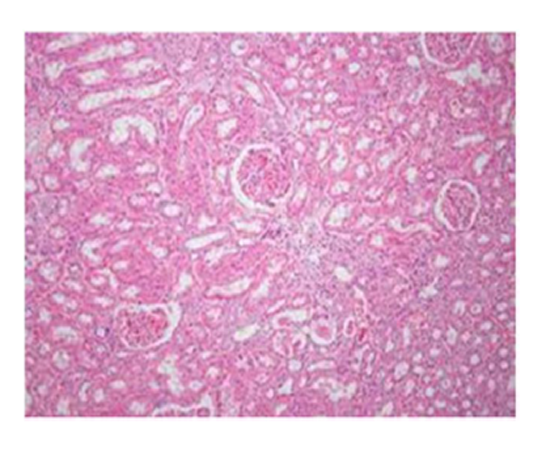
Group I & II: Microscopic examination of representative sections of kidney from group I (control) & group II (IA extract) showed normal histological appearance of the glomeruli and tubules. The proximal tubular epithelium was lined by eosinophilic cuboidal cells with intact brush border and well defined nuclei. They were arranged in a regular fashion over the basement membrane. The cell linings were intact with a patent lumen. No inflammatory cell infiltration or vascular changes were observed.
Suggestive: normal kidney.
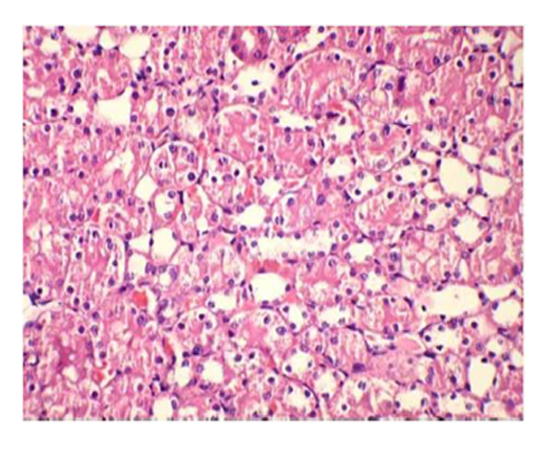
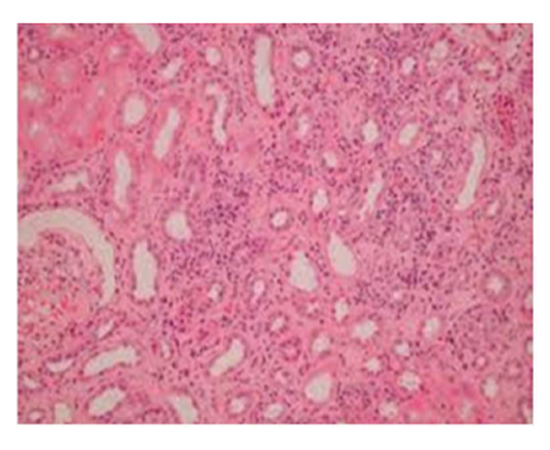
Group III: Representative photograph of sections of renal cortex under light microscope of rats group III showed glomerular congestion & necrosis in the proximal convoluted tubular. Interstitium showed infiltration with inflammatory cells.
Suggestive: massive interstitial nephritis.
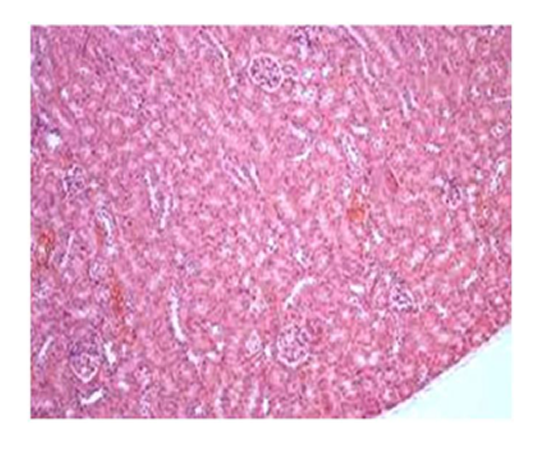
Group IV: Representative photograph of section of renal cortex under light microscope of group IV showed alleviation of renal architecture including glomeruli and tubules with interstitium infiltrated by moderate number of lymphocytes.
Suggestive: mild interstitial nephritis.
4. Discussion
Aminoglycoside including gentamicin (GM) can produce nephrotoxicity in human. Proximal tubular cells are a major site of damage in patients treated with GM [15]. GM binds to the cell wall phospholipids, blocking the chain reactions of phosphatidyl inositol which impairs cell integrity [16]. It has been shown that GM antibiotics exert their adverse renal effects by generation of reactive oxygen species (ROS). Formation of ROS following bioactivation of GM has been reported [17].
The present study showed that the administration of gentamicin on rats for 8 days reduces glomerular function, as reflected by increased serum creatinine & urea concentrations. Histopathology of kidney showed massive interstitial nephritis. Soliman et al. (2007), Gowrisri et al. (2012), Al-Majed et al. (2002) used gentamicin at dose 80 mg/kg for experimental nephrotoxicity on rats and they resulted similar to our study. Patil et al. (2010) applied gentamicin at dose 100 mg/kg for nephrotoxicity in rats and their results were similar to our study.
In this study it was observed that pre & concomitant administration of Ipomoea aquatica ethanol extract on GM-induced nephrotoxic rats reduced serum creatinine & urea concentration. In this research, histological observations of kidney of this group had also shown alleviation of renal architecture including glomeruli and tubules with interstitium infiltrated by a few number of lymphocyte. Therefore, the findings of this study are in well agreement with the findings of the other researcher Alqasoumi (2013). It may be concluded that pre & concomitant administration of IA extract on gentamicin induced nephrotoxic rats has been significantly prevented the renal injury both functionally and histological.
Several investigations showed that antioxidant agents inhibited or attenuated gentamicin induced nephrotoxicity on rats. Usage of antioxidants improved histological injuries such as tubular necrosis, tubular cell edema and apoptosis on gentamicin-injected rats [22, 23]. IA extract prevented renal cell injury by halting lipid peroxidation probably due to the antioxidant properties of extract exerted as inhibitory and scavenging action against reactive oxygen species (ROS) [24, 25].
Igwenyl et al. (2011) showed that different extract of Ipomoea aquatica contains alkaloids, steroids, saponins, phenols, reducing sugar, flavonoids, tannins, β- carotene, glycosides, cyanogenic glycosides and soluble carbohydrate. Flavonoids are a group of phytochemicals found in higher concentration from the ethanol extract of Ipomoea aquatica leaves, which have been shown to exert potent antioxidant activity against the superoxide radical [13].
So Ipomoea aquatica possess strong antioxidant properties, that is why it was used in present study with the expectation to interrupt the toxic free radical chain reaction of lipid peroxidation in the course of gentamicin administration by scavenging ROS, elevating antioxidant enzyme levels and thus oxidative damage to the renal cortex would be antagonized.
The results of biochemical and histological observations of the present study indicate that administration of Ipomoea aquatica extract was probably effective to counter the signs of toxic damage to the renal tubules.
5. Conclusion
The observation and result of this study provide the information that Ipomoea aquatica leaves have nephroprotective effect which requires further experiment. It is recommended that further studies regarding pharmacokinetics, pharmacodynamics, toxicology and posology of ethanol extract of Ipomoea aquatica leaves should be undertaken to develop it as a useful nephroprotective agent for human.
References
- Herfindal and Gourley, Text book of therapeutic drug and diseasemanagement, 7th ed. London: Charcil Livingstone, 2000, pp.425-36.
- Nissenson, A.R., Acute renal failure: definition and pathogenesis, Kidney Int Suppl. 66, 7–10 (1998).
- Naughton, C.A., Drug induced nephrotoxicity, Am Fam Physician. 78(6), 743-750 (2008).
- Ali B.H., Gentamicin nephrotoxicity in humans and animals: Some recent research, Gen Pharmacol. 26(7), 1477-87 (1995).
- Mathew T.H., Drug-induced renal disease. Med J.156, 724-28 (1992).
- SEAN C SWEETMAN, MARTINDALE, The Complete Drug Reference Thirty-sixth edition Published by the Pharmaceutical Press Lambeth High Street, London SEl 7JN, UK, , 2009.
- Balakumar, P., Rohilla, A. & Thangathirupathi, A., Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it, Pharmacoogical Research. 62, 179- 86 (2010).
- Lopez-Novoa J.M., Quiros Y., Vicente, L., Morales, A.I. & Lopez-Hernandez, F.J., New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view, Kidney Int. 79(1), 33-45 (2011).
- Balakumar, P., Chakkarwar, V.A., Kumar.V., Jain, A., Reddy, J. & Singh, M., Experimental models for nephropathy, J Renin Angiotensin Aldosterone Syst. 9 (4), 189-95 (2008).
- NITHA, B., JANARDHANAN, K.K., Aqueous-ethanolic extract of morel mushroom mycelium Morchella esculenta, protects cisplatin and gentamicin induced nephrotoxicity in mice. Food Chem Toxicol. 46, 3193-3199 (2008).
- NABAVI, S.F., EBRAHIMZADEH, M.A., NABAVI, S.M. & ESLAM, I.B., Antioxidant activity of flower, stem and leaf extracts of Ferula gummosa Boiss. Grasas Aceites. 61, 244-250 (2010).
- Nadkarni, K.M., Indian Materia Medica. Mumbai: Popular Prakashan. Vol. I, pp. 684 (1982).
- Huang,D., Chen,H., Lin,C. & Lin,Y., Antioxidant and antiproliferative activities of water spinach (Ipomoea aquatica Forsk) constituents, Bot Bull Acad Sin. 46, 99-106 (2005).
- Gowrisri, M., Kotagiri, S., Vrushabendra, S. B. M., Archana, S. P. & Vishwanath, K.M., Anti-oxidant and Nephroprotective Activities of Cassia occidentalis Leaf Extract against Gentamicin Induced Nephrotoxicity in Rats, Research Journal of Pharmaceutical, Biological and Chemical Sciences. July – September, 3 (3), 684-694 (2012).
- Wiland, P., & Szechcinski, J., Proximal tubule damage in patients treated with gentamicin or amikacin. Pol J Pharmacol. 55, 631–637 (2003).
- Walker, R.J., & Duggin, G.G., Drug nephrotoxicity. Annu Rev Pharmacol Toxicol. 28, 331- 345 (1988).
- Sha, S.H., & Schacht, J., Formation of reactive oxygen species following bioactivation of gentamicin. Free Radic Biol Med 26, 341–347 (1999b).
- Soliman, K.M., Abdul-Hamid, M., Othman, A.I., Effect of carnosine on gentamicin-induced nephrotoxicity. Med. Sci. Monit. 13(3): BR73-83 (2007).
- Al-Majed, A.A., Mostafa, A.M., Al-Rikabi, A.C., Al-Shabanah. O.A., Protective effects of oral arabic gum administration on gentamicininduced nephrotoxicity in rats. Pharmacol. Res. 46(5), 445-51 (2002).
- Patil, C.R., Jadhav, R.B., Singh, P.K., Mundada, S., Patil, P.R., Protective effect of oleanolic acid on gentamicin induced nephrotoxicity in rats. Phytother Res. 24(1), 33-7 (2010).
- Alqasoumi,S., Protective effect of Ipomea aquatica forsk on gentamicin-induced oxidative stress and nephropathy in rats, Topclass Journal of Herbal Medicine. 2 (2), 13-19 (2013).
- Tavafi, M., & Ahmadvand, H., Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats, Tissue Cell. 43 (6), 392-7 (2011).
- Baradaran, A., & Mahmoud, R.M., His¬topathological study of the combination of met¬formin and garlic juice for the attenuation of gen¬tamicin renal toxicity in rats, J Ren Inj Prev. 2 (1), 17-23 (2012).
- Kumar,V., Gogoi,B.J., Meghvansi, M.K., Singh, L., Srivastava, R.B. & Deka, D.C.,Determining The Antioxidant Activity Of Certain Medicinal Plants Of Sonitpur, (Assam), India Using Dpph Assay, Journal of Phytolog (1), 2075-6240 (2009).
- Prasad, K.N., Divakar. S., Shivamurthy, G.R. & Aradhya, S.M., Isolation of a free radical Scravenging antioxidant from water spinach (Ipomoea aquatica Forsk), Journal of Science, Food & Agriculture.85, 1461-1468 (2005).
- Igwenyl, I. O., Offor, C. E., Ajah, D. A., Nwankwo, O.C., Ukaomah, J. I. & Aja, P. M. Chemical composition of Ipomoea aquatica (Green Kangkong), 2, (4), 593-98 (2011) .