Information
Journal Policies
Ductal Carcinoma of the Prostate
Joaquin Juan Escudero1, Andrea Jimena Duran Rivera2*, Nuria Santonja3, Carlos Cayuelas1, Emilio Lopez1
2.Lluis Alcanyis hospital, Xátiva, Urology Department, Avenida Tres Cruces, 2, 46014, Valencia, Spain.
3.Valencia university general hospital, Pathological Anatomy Department, Spain.
Copyright : © 2019 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Ductal carcinoma of the prostate is a rare and aggressive histologic variant of prostate cancer. Frequently affects older patients and a metastatic debut are not uncommon. Although it is frequently a incidental diagnosis within the study of a suspected adenocarcinoma of the prostate, sometimes its local aggressiveness may provide some additional clues for the diagnosis.
spleen, splenomegaly, splenectomy, hairy cell leukemia,Clinical Case Reports
1. Introduction
Ductal carcinoma of the prostate is an infrequent histologic variant with an incidence between 0.2 to 0.8% of all prostate cancer variants.
It is originated from the periurethralis prostatic ducts and, less frequently, from the prostatic urethra. Even though it may coexist with the acinar cell adenocarcinoma, inmunohistochemical studies have considered the ductal carcinoma as a rare and independent histologic variant. Despite an evident similarity to adenocarcinoma, ductal carcinoma is more aggressive and metastatic debut is not uncommon. This is why, regardless of its histopathologic characteristics, it is always considered grade 4+4 in the Gleason classification.
2. Clinical Case 1
A 74 year-old man treated for a low risk, no musculoinvasive bladder cancer in 2002 with no disease recurrence and a PSA of 2,4ng/mL presents in August 2016 with acute urinary retention requiring an indwelling urinary catheter despite the fact that he was on alpha-blocker therapy. The patient underwent transurethral resection of the prostate (TUR-P) and the histologic report showed benign prostatic hyperplasia. One year later, PSA values started to rise up to 20ng/mL, a prostate biopsy was performed and the existence of ductal adenocarcinoma of the prostate in 10 out of 12 cores was reported.
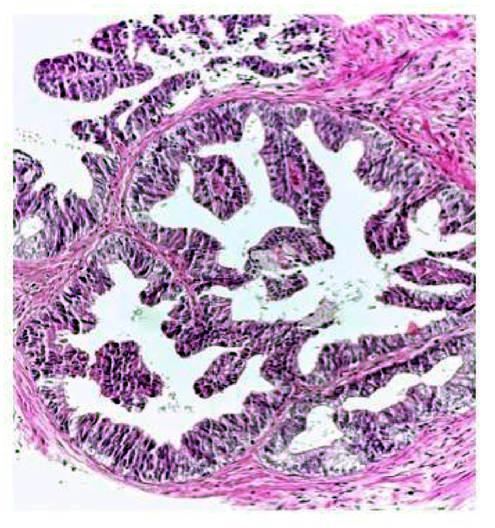
The histopathologic study showed proliferation of cribiform structures made of columnar cells in a pseudostratified epithelial distribution with moderated atypia. (Figure. 1).
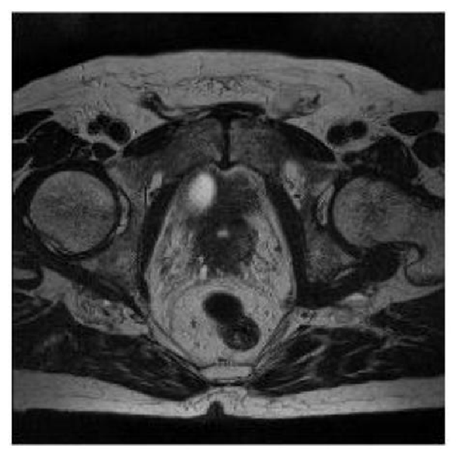
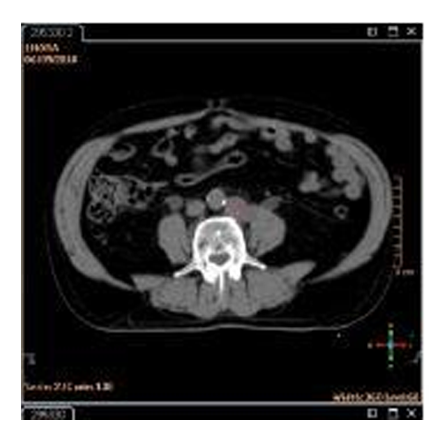
The clinical staging study was performed with a bone scan that showed a single metastatic lesion in T11 (Figure. 2) and multiparameteric magnetic resonance that showed extracapsular extension and infiltration of both seminal vesicles (Figure. 3).
According to the decision of the Uro-oncologic committee from our hospital, the patient was treated with image-guided radiation therapy of the prostate, seminal vesicles and pelvic lymphadenopathies, and with stereotactic body radiation therapy for the single bone metastasis.
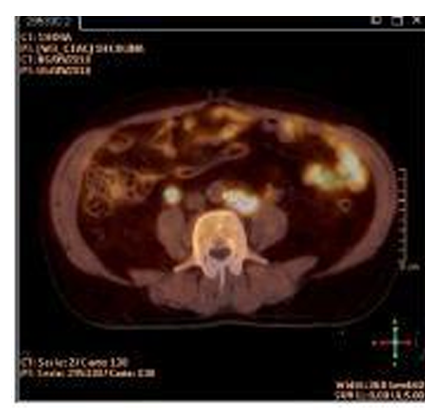
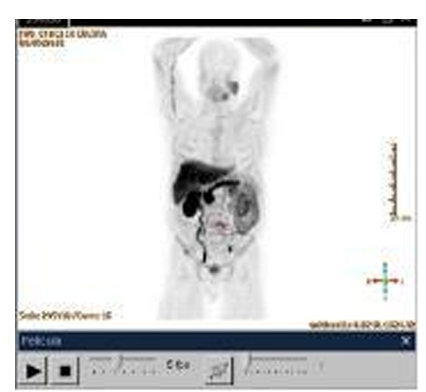
Due to the intrinsic aggressiveness of ductal carcinoma of the prostate, it is decided to extend the clinical staging evaluation with a PET-CT. The study showed positive lymphadenopathies in the common iliac, paraaortic and paracaval regions, as well as, two bone metastasis in the left iliac crest and the vertebral body of T11 (Figure. 4).
3. Clinical Case 2
A 76 year-old man with a past medical history of high blood pressure and TUR-P in 2013 with a histologic report of benign prostatic hyperplasia (PSA of 3,4ng/mL) is referred by his primary physician because a PSA rise of 10ng/mL in December 2015. A transrectal prostate biopsy is performed and revealed prostatic adenocarcinoma Gleason 4+3 in 4 out of 12 cores.
The bone scans showed no metastatic lesions and the MRI revealed the presence of a 17mm nodule PIRADS 5 infiltrating the prostate capsule.
In June 2016 the patient underwent radical prostatectomy with lymphadenectomy. The histopathologic report found two adjacent proliferations with different characteristics. The most extended one (90% of the neoplastic volume) consisted of cribiform, trabecular and tubular structures of cells with clear cytoplasm, and prominent nucleolus representing an acinar adenocarcinoma Gleason 4+5 with infiltration of the capsule without exceeding it (Figure. 5). The remaining 10% was a proliferation of papillary structures of cells with eosinophilic cytoplasm, bigger nucleus and prominent nucleolus, representing a papillary ductal carcinoma in the left prostate lobe (Figure. 6). All lymph nodes were negative. In the follow-up, PSA remains undetectable until today.
4. Discussion
It is uncommon to find a histologic diagnosis other than acinar cell adenocarcinoma in a prostate biopsy. Neuroendocrine carcinoma and ductal carcinoma are two of these exceptional variants with an estimated frequency of 0.2 and 0.8% among all prostate cancers.
Ductal carcinoma of the prostate frequently appears at an advanced age. In the presented cases, the peak age of incidence is in the seventh decade of life [1,2]. In fact, all published cases were around 75 years old by the time of the diagnosis.
This histologic variant grows form the epithelial cells of the periurethral prostatic ducts, and seldom from the prostatic urethra. Even though more than 50% of the cases are associated with conventional acinar cell adenocarcinoma [3], it may also appear isolated overshadowing the prognosis of the patient. It is frequent to find the diagnosis of ductal carcinoma of the prostate only in the histologic analysis of the entire prostate gland after radical surgery, while the prostate biopsy only shows acinar cell adenocarcinoma [4].
Although most cases of ductal carcinoma of the prostate are diagnosed as an incidental event after elevation of the PSA within the study and treatment for an acinar cell adenocarcinoma, given its intrinsic tendency to grow in an endophytic matter toward the urethral lumen, it is not infrequent for these patients to experience obstructive lower urinary tract symptoms or hematuria at early stages of the disease. This endophytic growth causes the diagnosis through rectal exam to be uncommon, and sometimes the PSA marker is not elevated [5]. Both of the presented cases had lower urinary tract voiding symptoms before the diagnosis. In neither of them the histologic analysis of the TUR-P samples showed ductal carcinoma, the only suggestion was the rising PSA.
This entity may also be suspected when endoscopic evaluation reveals a polypoid mass in the prostatic urethra. It may be papillary or cribriform with enlargement of the prostatic ducts [6]. However, none of these findings were present in our patients TUR-Ps.
Histologic diagnosis may be determined trough prostate biopsy or TUR-P. It is important to keep in mind differential diagnosis such as urethral tumor and bladder tumor when encountering polypoid lesions at the prostatic urethra or at the bladder neck level. There may be friable areas prone to bleeding by grazing them with the cystoscope at the level of the veru montanum, but sometimes, such as in the previously discussed cases, these findings may pass unnoticed in early stages.
These tumors usually have a cribiform pattern (especially if peripherally located) or a papillary pattern (especially if centrally located). They are often associated with fibrous stroma. There are other rare variants such as mucinous cells, Paneth type neuroendocrine cells, micropapillary or cystic papillary. At the inmmiunohistochemical level, the tumor cells are positive for PSA and PAP, and negative for high molecular weight cytokeratine (CK34BE12).
Regarding the histologic differential diagnosis, it is always crucial to take into account urothelial tumors, colon neoplasias, intraepithelial prostatic neoplasia, nephrogenic adenoma and the often concurrent acinar cell adenocarcinoma of the prostate. The high-grade intraepithelial prostatic neoplasia involves a differential diagnosis challenge but the nuclear size is bigger in ductal carcinoma [7].
By the time of the diagnosis, 12% of the cases of ductal carcinoma may present metastatic disease, being 3 times more frequent that metastatic debut in acinar cell adenocarcinoma [8], locally advanced disease at the time of presentation it is also more frequent. Metastatic tendency is similar to acinar, being the bones, lymph nodes and brain the usual metastatic places [1], but also ductal carcinoma has a greater tendency to compromise lungs and penis [2].
The 5-year survival rate of ductal carcinoma ranges between 15 and 45% [6,7], which results in a greater aggressiveness than the acinar cell adenocarcinoma justifying why it is approached and treated as a Gleason 4+4.
The treatment is similar to acinar cell adenocarcinoma, surgery or radiotherapy for the localized cases, being aware of the special aggressiveness that advocates lymphadenectomy for all patients. If radiotherapy is the selected treatment, local obstructive symptoms must be considered, and TUR-P must be considered as adjuvant. Finally, although it may be controversial, we consider that pelvic lymph nodes may also be treated in all cases.
While the follow-up of the patient that presented an advanced disease was short, the patient that presented with localized disease had a good oncologic result after the surgery. This makes us consider surgery as a great alternative for localized cases and even for locally advanced cases, always taking into account the possibility of a multimodal approach as adjuvant treatment.
5. Conclusion
Even though ductal carcinoma of the prostate is an infrequent entity, it must be taken into account for the differential diagnosis of patients with obstructive lower urinary tract symptoms. Although it has a more aggressive evolution that acinar cell adenocarcinoma, it can be cured in early stages.
References
- Senarriaga N, Loizaga A, Izaguirre R y cols. Carcinoma ductal del utrículo prostático. Caso Clínico y revisión de la literatura. Arch Esp Urol 2008; 61 (5): 637-640).
- Castañeda AM, Rufin AM, Gonzalez D, Diaz P, Almeida G y Sandoval FA. Adenocarcinoma ductal prostático. Una forma inusual de presentación del cáncer prostático. A propósito de un caso. Rev Pat Tocantis 2014; 1(4): 02-06.
- Picazo ML. Formas especiales de carcinoma de próstata. Patologia 1996; 29 (2): 153-7.
- Hayashi Y, KAwahara T, Iwashita H y cols. Ductal adenocarcinoma pof the prostate: a case report. Case Rep Oncol 2016;9:802–805.
- Colpaert C, GentensP, Van Marck E.. Ductal (“endometrioid”) adenocarcinoma of the prostate. Acta Urol Belg 1998;66:29–32.
- Ala Paredes MC, Garcia Serviche F, Roman Balderrama F, Canedo Garcia NA, Villarroel Salinas JC. Adenocarcinoma ductal de próstata tipo B: reporte de un caso Rev Cient Cienc Med 2014; 17(2):63-66.
- Fodor MF, Alonso EE. Carcinoma intraductal de la próstata. Una lesión con morfología similar a la neoplasia intraepitelial prostática, pero con un significado clínico diferente. Rev Med Rosario 2008; 74: 100-106.
- Morgan TM, Welty,CJ Vakar-Lopez F,Lin DW and Wright JL . Ductal Adenocarcinoma of the Prostate: Increased Mortality Risk and Decreased Serum Prostate Specific Antigen J Urol 2010; 184: 2303-2307.