Information
Journal Policies
Adenoid Cystic Carcinoma - A Case Report
Luciano Zogbi1*, Camila Juliano1, Fabiano Vendrasco1, Lorena Freitas1, Leonardo Porto1
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: Malignant cutaneous adnexal tumors are uncommon, primarily locally aggressive tumors that include adenoid cystic carcinoma, a rare skin adenocarcinoma.
Purpose: To present a case of adenoid cystic carcinoma and a brief literature review.
Case Report: A Caucasian male patient, 53 years old, presented with a 6-month history of a deep nodular lesion of about 3 cm in the malar region. Complete wide excision revealed an adenoid cystic carcinoma with characteristic findings that are described below. The patient had an adequate follow-up, without recurrences after 1 year postoperatively.
Conclusion: Malignant cutaneous adnexal tumors are rare, dangerous and potentially curative neoplasms; good prognosis depends on the ability of the physician to achieve a fast and accurate diagnosis.
Adenocarcinoma; Carcinoma, Adenoid Cystic; Skin neoplasms; Surgery,Clinical Case Reports
1. Introduction
Skin cancer has been the subject of worldwide concern because of its high incidence and its severe consequences if left untreated. Among the several types of skin cancer, malignant cutaneous adnexal tumors are rare, primarily locally aggressive tumors, composing fewer than 1% of cases. Adenoid cystic carcinoma (ACC) is a variant of adenocarcinoma that can arise from multiple organ types, including the skin. Reports of ACC are scarce in the literature [1–3]. The objective of the present study is to present a case of ACC and a brief review of the literature.
2. Case Report
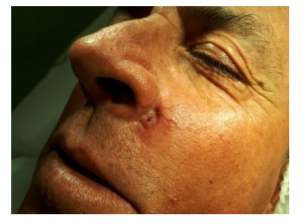
A 53-year-old Caucasian male, a Brazilian farmer, presented with a 6-month history of an asymptomatic, growing subcutaneous lesion in the left maxillary region next to the nasal wing. Physical examination revealed a nodular lesion about 3 cm in diameter, hardened and adherent to the deep and superficial planes, with a smooth surface and ulceration at the center with a hemorrhagic point as well as telangiectasias (Figure. 1). He denied other diseases.
He was underwent incisional biopsy, the histopathology of which revealed an undifferentiated malignant neoplasm. Immunohistochemical analysis was performed using the Leica Bond-Max Automated System, revealing positivity for the following markers: cytokeratin cocktail (clone AE1 and AE3), P63 (clone 4A4), EMA (clone E29), androgen receptor (SP 107), high-molecular-weight cytokeratin (clone 34βE12), cytokeratin 8 and low-molecular-weight cytokeratin (clone 35βH11). The lesion was negative for the following markers: monoclonal CEA (clone CEA31), cytokeratin 20 (clone IT-Ks20.8) and CD56 (clone 123C3.D5). This confirmed the diagnosis of carcinoma with a cutaneous origin, more precisely an ACC.
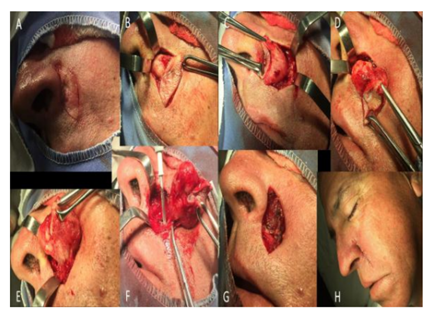
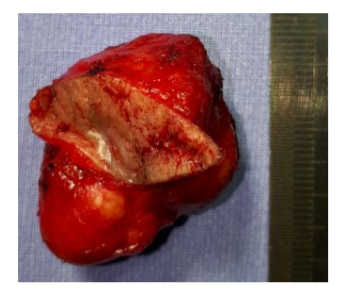
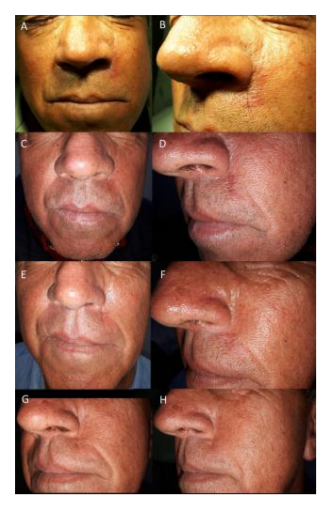
After staging to rule-out the possibility of metastases, the patient underwent definitive and complete resection of the lesion with safety margins (Figures. 2 and 3). Histopathological examination revealed a carcinoma with cutaneous adnexal origins, with extension to the deep reticular dermis, measuring 15.0 mm of maximum thickness, with moderate desmoplasia, moderate inflammatory mononuclear infiltrate and free surgical margins. One month after the procedure, the patient returned to the clinic without complaints or complications, and consultations were unrevealing. He completed more than one year postoperatively without recurrences (Figure. 4).
3. Discussion
Cutaneous adnexal tumors are a large group of benign and malignant neoplasms that exhibit morphological differentiation towards one of the four primary adnexal structures present in healthy skin: hair follicles, sebaceous glands, apocrine glands and eccrine glands [1]. Adnexal tumors represent a minority of cutaneous neoplasms. Data regarding their incidence and prevalence in the general population are lacking. With the exception of patients with syndromes associated with adnexal tumors and adnexal tumors arising in sebaceous nevi, most adnexal tumors occur in the fourth to the sixth decade [4], as was the case with this patient. Studying patients with malignant cutaneous adnexal tumors, Martinez found the median age at diagnosis was 70, with a slight male predominance. Lesions were located on the head and neck in 65% of cases. The 10-year disease-specific survival was 97% [5].
ACC is a variant of adenocarcinoma that can arise from multiple organ types. Salivary gland ACC is the most frequent type; however, primary ACC has been reported in skin, breast, lung, prostate, lacrimal gland, female genital tract and gastrointestinal tract [2]. Primary cutaneous ACC has a more favorable prognosis than does salivary ACC [3].
The tumor can occur on the scalp, trunk and extremities, typically presenting as a slow-growing, nondescript nodule on the skin. Diagnosis of ACC on the lower half of the face should prompt evaluation of local spread of salivary gland ACC.
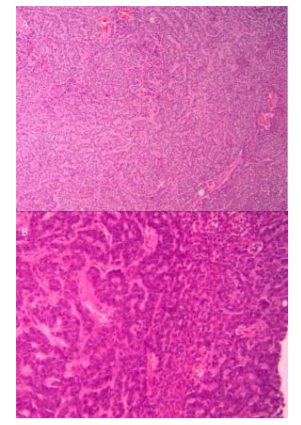
On histology, ACC is a malignant epithelial tumor consisting of modified myoepithelial and ductal differentiated cells. They are histomorphologically polymorphous but cytomorphologically they are relatively uniform, exhibiting three primary histopathologic growth patterns: cribriform, solid and tubular. The cribriform pattern is unique among these, with clusters of epithelial cells with both true ducts and duct-like collections of mucin interspersed in a sieve-like pattern. Solid and tubular patterns are also observed, with several patterns frequently present in the same tumor [6]. Our patient had a tumor infiltrating the dermis composed of ductal and glandular structures, with cribriform/acinar and solid arrangement. The morphologic growth patterns we observed had both a cribriform/ tubular and solid pattern. Nevertheless, the predominant growth pattern was cribriform.
Islands of neoplastic epithelial cells contained several small, circular and pseudocystic structures. Solid and cribriform patterns were both prominent in this tumor. The several tumor cells had amphophilic to eosinophilic cytoplasm and round-to-oval, lightly-stained nuclei with nucleoli; the nucleoli were not always evident (Figure. 5).
Patients diagnosed with malignant cutaneous adnexal tumors should undergo a complete skin examination that includes palpation of regional lymph nodes to evaluate for clinical signs of regional metastasis. If enlarged lymph nodes are detected, lymph node biopsy via fine needle aspiration or surgical removal of the enlarged lymph node is indicated. This was not the case in our patient, who did not present with enlarged lymph nodes on physical examination or imaging tests. The staging for malignant cutaneous adnexal tumors was based on the staging system for cutaneous squamous cell carcinoma in the 2017 eighth edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual [7].
Wide local excision with negative surgical margins or Mohs micrographic surgery is the recommended treatment for the majority of malignant adnexal tumors. Because of the rarity of these tumors, the width of safety margins has not been evaluated in clinical trials or large observational studies. In general, 1 to 2 cm clinical margins are used, which was the case in our patient; direct resection was chosen for the complete removal of the tumor in a block to achieve more radical excision. A retrospective review of 1045 patients with a histologic diagnosis of cutaneous adnexal carcinoma with eccrine differentiation showed no survival benefit to patients receiving adjuvant radiation therapy versus surgery alone. Radiation therapy should be reserved for patients for whom surgery is not an option or as a postsurgical adjuvant treatment when surgical margins cannot be cleared [8]. In the present case, because the surgery achieved the desired goal, i.e., removal of the entire tumor with free margins, we did not recommend radiotherapy.
This patient did not undergo sentinel lymph node biopsy because of the rarity of these tumors and the relatively low rate of nodal involvement, where the role of sentinel lymph node biopsy in patients without clinical lymph node involvement has not been established and cannot be recommended in routine practice[9].
Malignant cutaneous adnexal tumors are primarily locally aggressive tumors. Tumor recurrence resulting from incomplete removal of the tumor is a significant prognostic concern. Local recurrence rates range from 10 to 50 percent among patients treated with wide local excision or Mohs micrographic surgery. Routine postsurgical follow-up visits are advisable to monitor for recurrence [10]. The patient in this case completed one year of postoperative follow-up without relapses, but he will be followed periodically as an oncologic patient at risk for recurrence.
4. Conclusion
Malignant cutaneous adnexal tumors are rare, dangerous and potentially curative neoplasms that depend on the accuracy of the physician in an adequate and fast diagnosis.
References
- Obaidat N.A., Alsaad K.O., Ghazarian D., Skin adnexal neoplasms - part 2: an approach to tumours of cutaneous sweat glands. J. Clin. Pathol. 60(2), 145 (2007).
- North J.P., McCalmont T.H., Fehr A., et al., Detection of MYB Alterations and Other Immunohistochemical Markers in Primary Cutaneous Adenoid Cystic Carcinoma. Am. J. Surg. Pathol. 39(10), 1347 (2015).
- Ramakrishnan R., Chaudhry I.H., Ramdial P., et al., Primary cutaneous adenoid cystic carcinoma: a clinicopathologic and immunohistochemical study of 27 cases. Am. J. Surg. Pathol. 37(10),1603 (2013).
- Kazakov D.V., Kacerovska D., Hantschke M., et al., Cutaneous mixed tumor, eccrine variant: a clinicopathologic and immunohistochemical study of 50 cases, with emphasis on unusual histopathologic features. Am. J. Dermatopathol. 33(6),557 (2011).
- Martinez S.R., Barr K.L., Canter R.J., Rare tumors through the looking glass: an examination of malignant cutaneous adnexal tumors. Arch. Dermatol. 147(9), 1058 (2011).
- Weert S.V., van der Waal I., Witte B.I., Leemans R., Bloemena E. Histopathological grading of adenoid cystic carcinoma of the head and neck: Analysis of currently used grading systems and proposal for a simplified grading scheme. Oral Oncol. 51(1), 71-76 (2015).
- Part II Head and Neck. In: AJCC Cancer Staging Manual, 8th ed, Amid MB (Ed), Springer, New York 2017. p.53
- Avraham J.B., Villines D., Maker V.K., et al., Survival after resection of cutaneous adnexal carcinomas with eccrine differentiation: risk factors and trends in outcomes. J.Surg. Oncol. 108(1),57 (2013).
- Barnes M., Hestley A., Murray D.R., et al., The risk of lymph node involvement in malignant cutaneous adnexal tumors. Am. Surg. 80(3),270 (2014).
- Hamman M.S., Brian Jiang S.I.. Management of trichilemmal carcinoma: an update and comprehensive review of the literature. Dermatol. Surg. 40(7),711 (2014).