Information
Journal Policies
ARC Journal of Clinical Case Reports
Volume-3 Issue-1, 2017, Page No: 13-17
Acinar Cell Carcinoma of the Pancreas with Postoperative Lipase Hyper-Secretion Syndrome: Case Report and Review of the Literature
Walid Elhaj Abdelrahim 1*, Kamal Elzaki Elsiddig1, Mohamed Elfatih1, Mohamed Elhassan Akoad2, Bader Eldin Margani1, EAG Khalil3
1 Department of Surgery, Faculty of Medicine, University of Khartoum, Sudan.
2 Department of Transplantation, Lahey Clinic, Tufts University, USA.
3 Department of Clinical Pathology & Immunology, Institute of Endemic Diseases, University of Khartoum, Sudan.
2 Department of Transplantation, Lahey Clinic, Tufts University, USA.
3 Department of Clinical Pathology & Immunology, Institute of Endemic Diseases, University of Khartoum, Sudan.
Citation : Abdelrahim W E et al. Acinar Cell Carcinoma of the Pancreas with Postoperative Lipase Hyper-Secretion Syndrome: Case Report and Review of the Literature. ARC Journal of Clinical Case Reports. 2017;3(1):13-17.
Copyright : © 2017 Abdelrahim W E. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Lipase hyper-secretion syndrome (LHS) is a rare para-neoplastic syndrome that has been reported in association with acinar cell carcinoma (ACC). There are only a few LHS cases in the literature. A 68-year-old male presented with obstructive jaundice. ERCP findings showed distal common bile duct (CBD) stricture. Contrast-enhanced CT showed pancreatic head and uncinate process 6X5 Cm hypodense mass. Three weeks after admission, Whipple’s procedure and Child’s reconstruction were done. H&E and Immuno-histochemistry showed acinar cell carcinoma (ACC). On day three postoperatively the patient developed severe artharitis and inability to walk. Rheumatoid arthritis workup turned to be negative and uric acid level was within normal range. The diagnosis of lipase hypersecretion syndrome (LHS) was made retrospectively. Post-operative lipase hyper-secretion syndrome should be considered in patients with polyarthritis and negative rheumatoid workup in patients with the rare pancreatic acinar cell carcinoma.
Keywords: Acinar Cell Carcinoma, Lipase Hyper-Secretion Syndrome, Chemotherapy.
abbreviations: : ACC: acinar cell carcinoma, SMA: superior mesenteric artery, MV: superior mesenteric vein, DJ: duodeno-jejunal flexure, VIP: vasoactive intestinal peptide, PP: pancreatic polypeptide.
1.Introduction
Pancreatic acinar cell carcinoma (ACC) is a rare tumor that constitutes 1-2% of primary pancreatic neoplasm [1, 2]. ACC predominantly affects males with a mean age of 56 to 64 years at presentation [3]. ACC usually presents with abdominal pain, weight loss and less commonly jaundice in 12-20% of cases [4, 5]. A rare para-neoplastic syndrome called lipase hyper-secretion syndrome (LHS) was reported in association with ACC. The first case report of ACC of the pancreas associated with LHS was made in 1908 by Berner [6] who described a so-called functioning acinar cell carcinoma of the pancreas. The first appropriate interpretation of the patho-physiology was provided by Auger [7] in 1947. There are only a few case reports of LHS described in the literature [8-11]. LHS is characterized by poly-arthralgia, subcutaneous fat necrosis and esinophilia. ACC has earlier presentation, pancreas body and tail are more commonly affected and the disease is more localized compared to pancreatic adenocarcinoma (AC). The resection rate is higher even with large tumors and it ranges between 45-64% in comparison to < 10% in AC. Schmidt and colleagues [12] reported that stage-specific 5-year survival was significantly better for resected ACC compared to AC (stage I: 52.4% vs 28.4%; stage II: 40.2% vs 9.8%; stage III: 22.8% vs 6.8%; stage IV: 17.2% vs 2.8%). The preoperative diagnosis and clinical picture are non-specific. Although CT appearances in favor of ACC include: sizable, solitary, well-defined, heterogeneous hypo-dense mass with a well-defined enhancing capsule [13]. However, the diagnosis is usually by histopathology following resection in adjunct to Immunohistochemical staining. ACC cells show positive reaction with trypsin (100%), chymotrypsin (38%), lipase (77%), and amylase (31%) [14]. Pathogenesis of ACC is poorly understood, genetic and epigenetic genomic aberrations (e.g. TP53 gene and APC/β-catenin pathway alterations) have been suggested to play a role in ACC onset, pathogenesis and poor survival [2, 15, 16].
2.Case Scenario
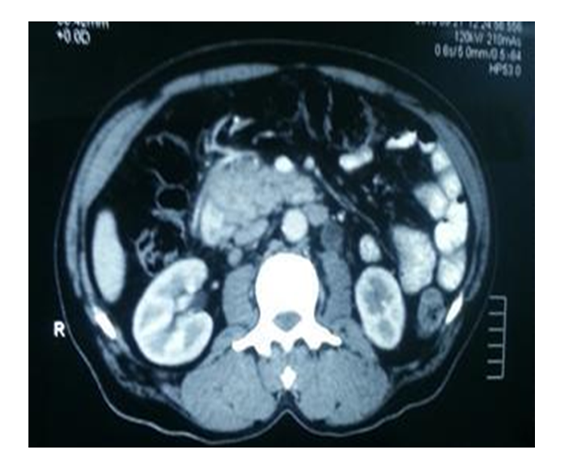
A 68-year-old married, male and ex-banker from a village, Gezira State, Central Sudan presented with epigastric pain for 3 months, yellowish discoloration of sclera for 2 months, dark urine, pruritus, and anorexia and weight loss. ERCP showed: distal common bile duct (CBD) stricture. A stent 10 Cm, 7 FR was deployed to the CBD. He denied any past history of jaundice, blood transfusion or surgical operations. He was hypertensive for >10 years on Lisinopril, 10 mg once daily. In addition, to intermittent arthralgia for 3 years labeled as gouty arthritis (on allopurinol 100 mg/daily). He developed pulmonary edema 3 years ago and was on Furosemide 40 mgdaily). On examination, he was pale, not jaundiced and no signs of chronic liver disease were elicited. Cardiovascular system and Chest were clear. Abdominal examination: showed no ascites or palpable masses. Hb was 99 grams/L, platelets count of 227×103 µ/L, esinophils 0.3 ×103 µ/L. Serum creatinine was 0.9 mg/dl, serum of K+ 2.8 mmol/L, serum Na+ 140 mmol/L, INR 1.02. Albumin 37 grams/L, total bilirubin 0.8 mg/dl, ALP 135U/L, AST/ALT 10/16.8 IU/L and serum carbohydrate antigen (CA) 19-9 0.4 ng/dl. US examination of the abdomen showed a mass in the pancreatic head, probably carcinoma. Dilated intra and extrhepatic biliary tree and CBD [12 mm]. CT scan showed a pancreatic head and uncinate process hypo-dense mass [6×5 –cm], with 180º abutment of SMV and SMA. The first jejunal branch was lifted by the tumor (Figure 1). As routine hospital practice the patient was consented for surgery. Surgery findings were negative for ascites, liver metastases, lymphadenopathy and peritoneal seedlings. A small 1X2 Cm nodule in the proximal jejunum close to the dudeno-jejunal (DJ) flexure was seen. A well circumscribed, firm and whitish 5X4 Cm pacreatic head mass was seen. The mass involved the uncinate process and it was separable form the SMA and SMV. A Whipple’s procedure and Child’s reconstruction were accomplished. Drains inserted for serial amylase assessment on days 3 and 5 with the following results: 181 and 30 IU/L respectively. Oral intake was initiated on day5 postoperatively. Drained were removed on day 6. Histopathology results of a 6x5.5 X 3 cm pancreatic tissue with surrounding small intestine, showed pancreatic acinar cell carcinoma.
On day three postoperatively the patient developed bilateral knees, ankles and hands small joints’ pain and swelling to the extent that he couldn't get out of bed. The Rheumatologist assessed the patient and the Rheumatology work up was negative [anti-CCP 7.4 U/L, RF 65.6 IU/L, ESR 5 mm/hrs, serum uric acid 5.1 mg/dL]. Diclofenac sodium 75mg therapy was initiated. The patient was assessed for LHS after the histopathology result and the lipase level was normal day 6 post operatively [40 U/L]. The patient was discharged on day 8 post-operatively and was symptom-free when he was seen a month after resection. The patient received 12 sessions of Gemcitabine-based chemotherapy.
This report aims to highlight the importance of maintaining high index of suspicion for this rare syndrome in patients with acinar cell carcinoma.
3.Discussion
Pancreatic ACC is a rare malignant epithelial neoplasm that exhibits exocrine enzyme production. LHS occurs only in 10-15% of patients with ACC. This para-neoplastic syndrome shows elevated serum lipase activity and peripheral blood eosinophilia associated with clinical signs of subcutaneous fat necrosis and polyarthritis. Less commonly osteolytic lesions have been described. Serum amylase activity is increased in only 30% of cases of LHS [16, 17]. Our patient had severe arthritis with negative rheumatology work up and his lipase level was normal on day 6 postoperatively. However, his symptoms completely resolved one month after resection.
Currently, surgical resection is the only curative approach to pancreatic ACC [18, 19]. Kitagami and colleagues [19] reported that the 5-year survival rate for patients with resected ACC was 43.9%, with a median survival time (MST) of 41 months. On the other hand, the 5-year survival rate for un-resected cases was 0%, with a MST of 3 months. Although there is no doubt that surgery is the mainstay for the treatment of ACC, a high recurrence rate of 72% following surgical resection has been reported. Therefore, effective chemotherapy is necessary to improve the clinical outcome. However, chemotherapy regimens have not been established due to the rarity of this disease [19, 20]. A study carried out in the Memorial Sloan-Kettering Cancer Center of 18 ACC patients who received in-homogeneous chemotherapy regimens, containing Gemcitabine, 5-FU, Leucovorin, Mitomycin, Cisplatin, Cytarabine, Irinotecan, Caffeine and Doxorubicin, reported partial responses (PRs) and stable disease in some patients. It is clearly evident that a protocol for the ACC adjuvant chemo-therapy is highly needed [21].
4. Conclusion,
post-operative lipase hyper-secretion syndrome should be considered in patients with polyarthritis and negative rheumatoid workup in patients with the rare pancreatic acinar cell carcinoma.
5.Ethical and Publication Approval
Ethics approval was obtained from the Ethics Committee of the Faculty of Medicine, University of Khartoum, Sudan.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Matos JM, Schmidt CM, Turrini O, Agaram NP, Niedergethmann M, Saeger HD, Merchant N, Johnson CS, Lillemoe KD, Grützmann R. Pancreatic acinar cell carcinoma: a multi-institutional study. J Gastrointest Surg 2009; 13.8: 1495-1502.
- La Rosa S., Sessa F., Capella C. Acinar Cell Carcinoma of the Pancreas: Overview of Clinicopathologic Features and Insights into the Molecular Pathology. Front Med (Lausanne). 2015; 2: 41. Published online 2015 Jun 15. doi: 10.3389/fmed.2015.00041.
- Seth AK, Argani P, Campbell KA, Cameron JL, Pawlik TM, Schulick RD, Choti MA, Wolfgang CL.. Acinar cell carcinoma of the pancreas: an institutional series of resected patients and review of the current literature. J Gastrointest Surg 2008; 12:1061–1067.
- Wisnoski NC, Townsend CM Jr, Nealon WH, Freeman JL, Riall TS. Patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery 2008; 144.2: 141-148.
- Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka MAcinar cell carcinoma of the pancreas: Clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas 2007; 35:42–46.
- Berner Subkutane Fettgewebsnekrose . Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medizin S1908; 193 (3): 510-518.
- Auger C. Acinous cell carcinoma of the pancreas with extensive fat necrosis. Arch Pathol 1947; 43:400–405.
- Potts DE, Mass MF, Iseman MDSyndrome and pancreatic disease, subcutaneous fat necrosis and polyserositis: Case report and review of literature. Am J Med 1975; 58:417–423.
- Radin DR, Colletti PM, Forrester DM, Tang WW, Pancreatic acinar cell carcinoma with subcutaneous and intraosseous fat necrosis. Radiology 1986; 158:67–68.
- van Klaveren RJ, de Mulder PH, Boerbooms AM, van de Kaa CA, van Haelst UJ, Wagener DJ, Hafkenscheid JC. Pancreatic carcinoma with polyarthritis, fat necrosis, and high serum lipase and trypsin activity. Gut 1990; 31: 953–955.
- Wisnoski NC, Townsend CM Jr, Nealon WH, Freeman JL, Riall TS. 672 patients with acinar cell carcinoma of the pancreas: A population-based comparison to pancreatic adenocarcinoma. Surgery 2008; 144:141–148.
- Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY.. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg 2008; 12:2078–2086.
- Chiou YY, Chiang JH, Hwang JI, Yen CH, Tsay SH, Chang CY.. "Acinar cell carcinoma of the pancreas: clinical and computed tomography manifestations. J Comput Assist Tomogr 2004; 28 (2): 180-6.
- Morohoshi T, Kanda M, Horie A, Chott A, Dreyer T, Klöppel G, Heitz PU.l.) Immunocytochemical markers of uncommon pancreatic tumors: Acinar cell carcinoma, pancreatoblastoma, and solid cystic (papillary-cystic) tumor. Cancer 1987; 59:739–747.
- Furlan D, Sahnane N, Bernasconi B, Frattini M, Tibiletti MG, Molinari F, et al. APC alterations are frequently involved in the pathogenesis of acinar cell carcinoma of the pancreas, mainly through gene loss and promoter hypermethylation. Virchows Arch. (2014); 464 (5):553-64. doi: 10.1007/s00428-014-1562-1. Epub 2014 Mar 4.
- La Rosa, S., Bernasconi, B., Frattini, M., Tibiletti MG., Molinari F., Furlan D. et al., TP53 alterations in pancreatic acinar cell carcinoma: new insights into the molecular pathology of this rare cancer. Virchows Arch (2016) 468: 289. doi:10.1007/s00428-015-1882-9
- Holen I, Croucher PI, Hamdy FC, Eaton CL Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res 2002; 62:1619–1623.
- Burns WA, Matthews MJ, Hamosh M, Weide GV, Blum R, Johnson FB. Lipase-secreting acinar cell carcinoma of the pancreas with polyarthropathy: A light and electron microscopic, histochemical, and biochemical study. Cancer 1974; 33:1002–1009.
- Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka M. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas 2007; 35:42–46.
- Distler M, Rückert F, Dittert DD, Stroszczynski C, Dobrowolski F, Kersting S, Grützmann R. Curative resection of a primarily unresectable acinar cell carcinoma of the pancreas after chemotherapy. World J Surg Oncol 2009; 7:22.
- Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, Saltz LB. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol 2002; 20: 4673–4678.