Information
Journal Policies
Langerhans Cell Histiocytosis Co-Existing with Papillary Carcinoma of Thyroid - A Rare Surgical Challenge
Jaimanti Bakshi1*, Joshi Kiran2
2.Senior Resident, Department of Otolaryngology & Head Neck Surgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Copyright : © 2018 Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Langerhans cell histiocytosis (LCH) is a rare disorder of unknown origin characterized by infiltration of the involved tissues by large numbers of Langerhans cells, often organized into granulomas. Here we report a case of Langerhans cell histiocytosis associated with papillary thyroid carcinoma (PTC) in a 31 year old man who presented with an enlarged, diffusely firm, non tender, non mobile thyroid swelling. Fine needle aspiration cytology showed involvement of thyroid by langerhans cell histiocytosis. Patient was evaluated, and no evidence of systemic involvement was found. Patient received ten cycles of chemotherapy with vinblastine and etoposide, no decrease in size of swelling was noted. Subtotal thyroidectomy was performed. Histopathologic study revealed Langerhans cell histiocytosis along with papillary carcinoma in biopsy tissue. After histopathologic confirmation, patient was subjected to radioiodine ablation therapy.
Diagnostic and Clinical, Utility of Immunohistochemistry,Papillary Thyroid Carcinoma,Cancer Science
1.Introduction
Langerhans cell histocytosis (LCH) is a group of clinical conditions of unknown etiology characterized by clonal proliferation of bone marrow Langerhans like cells either in situ, leading to isolated bone lesions or, having spread in different remote tissues, potentially capable of inducing multisystem disease. Sometimes there are mutations (BRAF gene)[1] in Langerhans cells as they form. These changes may make the Langerhans cells grow and multiply quickly. This causes LCH cells to build up in certain parts of the body, where they can damage tissue or form lesions. Historically, the disease was confirmed by showing birbeck granules by electron microscopy, but currently the diagnosis is based on immunostaining showing presence of CD1a and / or langerin. Papillary thyroid carcinoma (PTC) is a well differentiated epithelial neoplasm. It is more common in women. Although it affects all age groups, the mean age at diagnosis is 40 years. Prognosis is good in patients aged ≤45 years (more than 90%). PTCs manifest themselves as nodules, and diagnosis relies on patient‟s clinical parameters, ultrasonography, and fine needle aspiration cytology. Few cases have been reported of thyroid gland involvement coexsistently by LCH and Papillary carcinoma. We do present a case of thyroid involvement by LCH and papillary carcinoma coexsistently with diagnostic controversy between aspiration cytology and histopathology.
2.Case Report
A 31years old man presented in otolaryngology outdoor with complaint of swelling in anterior part of neck for past two years which was insidious in onset and gradually progressive. He also had complaints of polyuria and polydypsia for past six months and has been diagnosed with diabetes insipidus and this disorder was controlled by desmopressin nasal spray.
His other health problems included diabetes mellitus, hypothyroidism and thalassemia trait which were well controlled with medical treatment.
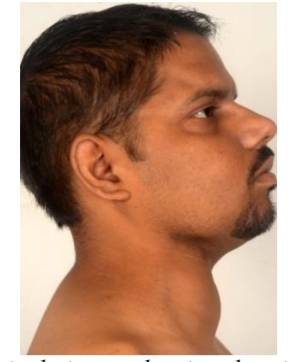
Physical examination of the patient revealed approximately 7cmx8cm firm swelling in right lower part of neck which moved with deglutition (Figure 1). The swelling was non tender, non pulsatile with restricted mobility. There were no abnormal findings in ear, nose and throat.
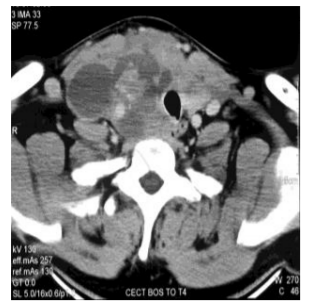
A fine needle aspiration cytology showed presence of langerhans cell histiocytosis which was CD1a positive on immunostain. Contrast enhanced computed tomography scan of the neck (Figure 2) revealed heterogeneous enlargement of right thyroid lobe (10.4x6.4x8 cm) with retrosternal extension. Right common carotid artery was encased by the mass. Left lobe of thyroid was normal.
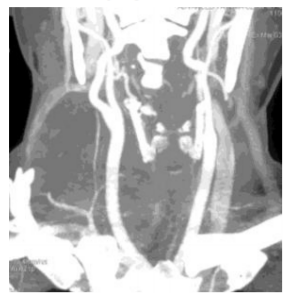
CT Angiography neck (Figure 3) showed hypodense mass in right lobe of thyroid encasing distal brachiocephalic trunk and proximal segment of right common carotid artery circumferentially. Right IJV was not visualized along the course of the lesion with dominant drainage into the external and anterior jugular veins.
Magnetic resonance imaging of the brain, nuclear bone scan, and chest X ray were normal. He had received chemotherapy with vinblastin and etoposide but no reduction in size of swelling was noted.Patient developed neutropenia and drug induced colitis as a side effect which was controlled by medical treatment.
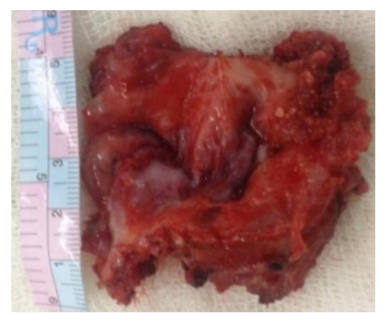
Surgical excision was planned under GA. Intraoperative findings (Figure 4) revealed 10x8cm tense cystic swelling involving right lobe of thyroid, isthmus and left lobe. The cyst was circumferentially encasing the right internal juglar vein and adhered to anterior wall of right common carotid artery. We performed subtotal thyroidectomy. Right internal juglar vein was ligated. Cyst wall adherent to anterior wall of common carotid was left in situ. Patient recovered from anesthesia without any complications. The post operative period was uneventful.
Histopathology revealed papillary arrangement of tumor cells with nuclear crowding, overlapping, grooving and optical clearing. Few aggregates of langerhans cells with vesicular cleared nuclei were also noted which were highlighted on CD1a immunostain. Focal giant cell reaction and calcifications were also identified along with moderate lymphoplasmacytic infiltrate admixed with neutrophils and eosinophils.
After histopathologic confirmation of coexistent papillary thyroid carcinoma and langerhans cell histiocytosis, patient was subjected to radioiodine ablation therapy. Patient is disease free for 6 months after treatment.
3. Discussion
Langerhans cell histiocytosis is a rare disease. In 1953 Lichtenstein coined the term "histiocytosis X" to encompass three disease entities of langerhans cells including eosinophilic granuloma of bone, Hand Schüler Christian disease (the triad of exophthalmos, diabetes insipidus and multiple bony lesions) and Letterer Siwe disease (a syndrome of rapid, progressive multiple soft tissue involvement)[2]. In fact, there is a considerable overlap between these entities.
LCH usually presents as a multisystem granulomatous infiltrate (in two thirds of cases) in adults [3]. The condition typically presents with the involvement of bone, lung, skin, lymph nodes, and multiplesites [4]. Most common endocrinological manifestation of classical LCH is associated with posterior pituitary involvement presenting as diabetes insipidus [5].However, LCH involving the thyroid gland is extremely rare [6]. Thyroid involvement of LCH can be indistinguishable from other thyroid disorder presenting with goiter. It is important to distinguish isolated thyroid LCH from multisystemic cases because single organ involvement is associated with an excellent survival of close to 100% [7]. Thyroidal diseases secondary to LCH may present as thyroid enlargement because of infiltration of the thyroid by LCH [8].
They do demonstrate a characteristic nuclear groove in histopathology and positive immuno staining with CD68, S100, and CD 1a as in our case [9].
Under the recommendation of the Writing Group of the HistiocyteSociety, [10] a definitive diagnosis of LCH can only be rendered when either there is demonstration of Birbeck granules on electron microscopic study or there is the demonstration of CD1a expression with immunohistochemistry, the latter being present in our case.
Due to its rarity, diagnosing LCH of the thyroid can be challenging. It can be clinically confused with the far more common benign goiters; un differentiated carcinoma, lymphoma, lymphocytic thyroiditis, and chronic granulomatous thyroiditis.
Langerhans cell histiocytosis of the thyroid may occasionally be associated with a nor or with hypothyroism as in our patient [11].
The role of chemotherapy in histiocytosis X is controversial[12]. There have been no trials large enough to confirm the benefit of a specific regime.
Multiple chemotherapeutic regimens have been attempted: glucocorticoids, vinblastine, etoposide, cyclophosphamide, methotrexate, and doxorubicin. Our patient had also received chemotherapy with vinblastine and etoposide but no reduction in size of swelling was noted.
Surgical treatment is the suitable option. For LCH localized to the thyroid, resection of LCH by subtotal, near total, or total thyroidectomy is the treatment of choice.
Papillary thyroid carcinoma is the most common thyroid malignancy with a high rate of BRAF V600E mutations [13]. Most commonly found in younger (< 45years old) patients. Histologically, it consists of epithelial papillary projections between which calcified spherules (psammoma bodies) may be present. Nuclei are characteristically large with central clear areas (orphan Annie nuclei). Papillary carcinoma metastasizes via the lymphatics to the cervical lymph nodes. Diagnosis of papillary carcinoma relies on patient's clinical parameters, ultrasonography, and fine needle aspiration cytology.
LCH is known to be associated with other neoplasms, such as lymphoma, leukemia or lung cancer,[14] but the association with PTC remains rare and unclear, as there have only been a few reported cases. However, a recent study showed an increased presence of CD1a positive dendritic cells in PTC[15].The association of LCH with PTC, which is rare, can be attributed to the fact that the same mutation may play a role in the underlying pathogenesis of both diseases.
Thyroid involvement in LCH is rare, as is its association with PTC. Surgical removal seems to be the gold standard treatment for isolated LCH, where as disseminated disease requires systemic therapy. Associated PTC may require further treatment, such as radioiodine therapy, but there are no standard recommendations.
In summary, in patients with thyromegaly, the possibility of Langerhans cell histiocytosis, although rare, should be kept in mind. A careful microscopic examination, immunohistochemical and ultrastructural studies are very important to make a correct diagnosis. In view of the increasing incidence of PTC, especially in women, one possible clinical implication of these findings is that patients with LCH characterized by activating BRAF mutations should be monitored for PTC.
References
- Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16(12):1229-42.
- Baloch ZW, LiVolsi VA. Pathologic diagnosis of papillary thyroid carcinoma: today and tomorrow. Expert review of molecular diagnostics. 2005; 5(4):573-84.
- Papale F, Cafiero G, Grimaldi A, Marino G, Rosso F, Mian C, et al. Galectin‐3 expression in thyroid fine needle cytology (t‐FNAC) uncertain cases: Validation of molecular markers and technology innovation. Journal of cellular physiology. 2013; 228(5):968-74.
- Weber T, Schilling T, Büchler MW. Thyroid carcinoma. Current opinion in oncology. 2006; 18(1):30-5.
- Saleh HA, Feng J, Tabassum F, Al-Zohaili O, Husain M, Giorgadze T. Differential expression of galectin-3, CK19, HBME1, and Ret oncoprotein in the diagnosis of thyroid neoplasms by fine needle aspiration biopsy. Cytojournal. 2009; 6(1):18.
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nature reviews Molecular cell biology. 2010;11(7): 502-14.
- Klezovitch O, Vasioukhin V. Cadherin signaling: keeping cells in touch. F1000Research. 2015; 4(F1000 Faculty Rev).
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Developmental cell. 2006; 11(5):601-12.
- Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. The Journal of cell biology. 2000; 148(4):779-90.
- ElMoneim HMA, Zaghloul NM. Expression of E-cadherin, N-cadherin and snail and their correlation with clinicopathological variants: an immunohistochemical study of 132 invasive ductal breast carcinomas in Egypt. Clinics. 2011; 66(10):1765-71.
- Sipos J, Mazzaferri E. Thyroid cancer epidemiology and prognostic variables. Clinical oncology. 2010; 22(6):395-404.
- Hedinger C, Dillwyn Williams E, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989; 63(5):908-11.
- El-Foll H, El-Sebaey H, El-Kased A, Hendawy A, Kamel M. Pattern and Distribution of Lymph Node Metastases in Papillary Thyroid Cancer. J Clin Exp Pathol. 2015;5(204):2161-0681.
- Bremmer F, Hemmerlein B, Strauss A, Burfeind P, Thelen P, Radzun H-J, et al. N-cadherin expression in malignant germ cell tumours of the testis. BMC clinical pathology. 2012; 12(1):19.
- Yang X, Shi R, Zhang J. Co-expression and clinical utility of Snail and N-cadherin in papillary thyroid carcinoma. Tumor Biology. 2016;37(1):413-7.
- Da C, Wu K, Yue C, Bai P, Wang R, Wang G, et al. N-cadherin promotes thyroid tumorigenesis through modulating major signaling pathways. Oncotarget.2017;8(5):8131.
- Zhuo H, Jiang K, Dong L, Zhu Y, Lü L, Lü Y, et al. Overexpression of N-cadherin is correlated with metastasis and worse survival in colorectal cancer patients. Chinese Science Bulletin. 2013; 58(28-29):3529-34.
- Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M, et al. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clinical Cancer Research. 2004; 10(12):4125-33.
- Lascombe I, Clairotte A, Fauconnet S, Bernardini S, Wallerand H, Kantelip B, et al. N-cadherin as a novel prognostic marker of progression in superficial urothelial tumors. Clinical Cancer Research. 2006; 12(9):2780-7.